How the heart fires up
Muscle contraction is a fundamental process enabling movement and force generation in the human body. At its core, contraction is driven by the interaction of two protein filaments, myosin and actin. Myosin functions as a molecular motor, generating force by binding to actin filaments and pulling them in a process known as the cross-bridge cycle. This interaction is regulated primarily by calcium ions, which control the exposure of myosin-binding sites on actin.
Dual regulation of muscle contraction
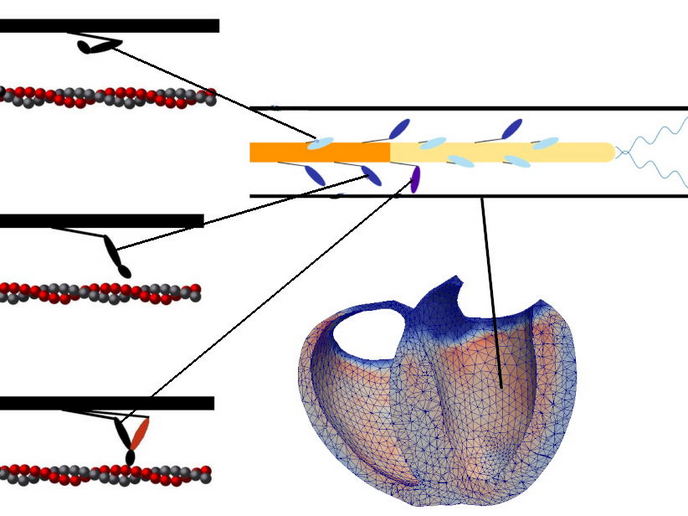
Accumulating evidence indicates a secondary regulatory mechanism influencing myosin availability, introducing new insights into muscle function. This pathway involves the transition of myosin between an active and an inactive state. Undertaken with the support of the Marie Skłodowska-Curie Actions programme, the Heart Fi-Re project was designed to deepen our understanding of heart muscle contraction by exploring the two regulatory pathways. In its inactive state, beside its classical “ready-to-go” state, where force generation depend solely on the activation of actin filament, myosin can also exist in a metabolically low-energy conformation known as the super-relaxed state (SRX). In this state, myosin remains detached from actin and consumes minimal adenosine triphosphate, reducing its availability for force-generating processes. The tension generated by active myosin provides feedback that ‘wakes up’ SRX myosin, allowing the participation to the force-generating cycle. Understanding these intertwined regulatory pathways was the primary focus of the Heart Fi-Re project.
Key findings
As the MSCA research fellow Lorenzo Marcucci explains: “We wanted to create a more precise model of how myosin transitions between different states, integrating molecular understanding into a broader simulation that connects single-molecule activity to the function of an entire heart.” By collaborating with a cluster of researchers with complementary expertise, Heart Fi-Re was able to differentiate between the biochemical and structural states of myosin. Previously, the SRX state was thought to correspond directly with a structurally inactive state where myosin lies along its filament backbone. However, new evidence suggested that these two states do not always overlap, necessitating a reassessment of existing models. Additionally, a mathematical model was developed to analyse calcium diffusion and its interaction with myosin activation, further elucidating the relationship between the two regulatory pathways. This model is now being refined and expanded with new experimental data, with the goal of incorporating it into personalised medicine strategies.
Clinical impact and future applications
The discoveries made through the Heart Fi-Re project hold significant potential for clinical applications. Within just a few years of its discovery, the SRX state became the target of an innovative drug therapy for hypertrophic cardiomyopathy, a genetic heart disease affecting approximately 1 in 500 individuals worldwide. The project’s integration of experimental data into a multi-scale heart simulator is a crucial step towards personalising treatment approaches and optimising therapeutic outcomes. The knowledge gained through this project may also extend beyond cardiac applications. Given that myosin regulation plays a vital role in skeletal muscle function, these findings could inform treatments for various muscle-related disorders. “The next phase of research will focus on validating the multi-scale heart simulator by comparing healthy and pathological data, pre- and post-treatment, in patients receiving drugs targeting myosin regulation. If successful, this model could guide clinical decision-making, including precise dosage estimations tailored to individual patients,” concludes Marcucci.
Keywords
Heart Fi-Re, myosin, muscle contraction, actin, SRX, regulation, calcium