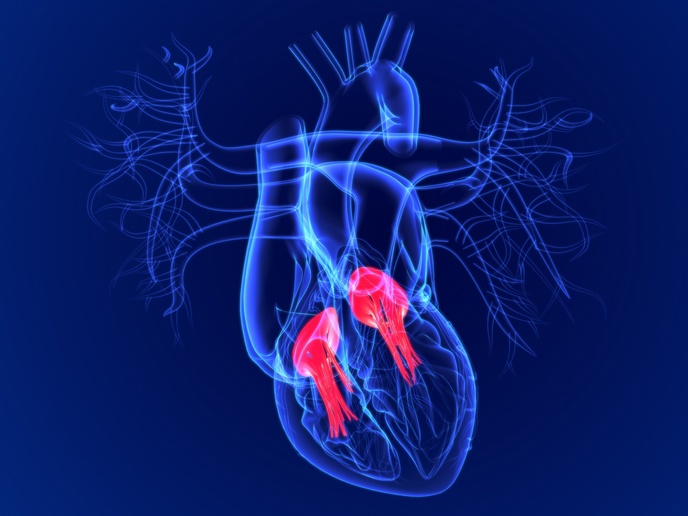
Catching up with TRICARIX: Helping tricuspid valve patients the minimally invasive way
Launched in 2018 and coordinated by TRiCares in Germany, TRICARIX set out with an ambitious goal: to make open-heart surgery unnecessary for patients suffering from severe tricuspid regurgitation, a disease in which the valve between the two right heart chambers does not close as it should. The solution proposed was a transcatheter tricuspid valve replacement system called Topaz that TRiCares continued to develop after the project’s end in 2022.
Benefiting patients on two continents
Since then, clinical trials have been carried out and 16 patients have been successfully treated in the project’s official study. “We also frequently receive requests from cardiologists who have patients that cannot be treated with any other device, but are suitable for the TRiCares Topaz system,” remarks Wolfhard Pinkowski, Head of Quality and Regulatory Affairs at TRiCares. He adds: “We are happy to help these patients through compassionate use programmes.” So far, another 40 patients have been successfully treated through such programmes across Europe and Canada. In May 2024, the project team also received approval from the U.S. Food and Drug Administration for an early feasibility clinical study in the United States that is expected to start within the year. In September 2024, in a European Pivotal Study (TRICURE) demonstrating the valve replacement system’s safety and effectiveness, TRiCares successfully performed the first Topaz implantation in an 81-year-old female patient in Belgium. Additional implantations are planned in Germany, Belgium, France and Switzerland, with additional countries expected to follow. The current catheter in-out time, an important parameter in the implantation process, is under half an hour. This is an impressive time when compared with that needed for other tricuspid devices currently under development or on the market. TRICARIX’s success inspired further developments. Using the one small size of a valve developed under the project as a springboard, TRiCares decided to upscale the implant, also developing a new larger size whose first human application is now in the works. Once this larger size is approved, TRiCares valves will be able to cover almost 90 % of patients suffering from tricuspid regurgitation. These achievements were made possible thanks to EU funding, helping to finance the project in its early and risky development phase. This initial support has also helped boost the company’s reputation, most recently evidenced by the fact that TRiCares was able to raise EUR 46 million in Series D funding from a strategic investor.
Keywords
TRICARIX, heart, valve, tricuspid, tricuspid regurgitation, catheter, valve replacement