Researchers improved muscular dystrophies by making the muscle slower
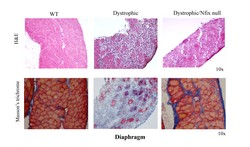
Patients with MD, such as Duchenne muscular dystrophy, suffer from dystrophy or wasting of skeletal muscle, which compromises movement. At worst, breathing and heart function is affected leading to wheelchair dependency, respiratory failure and premature death. There is still no therapeutic solution for these severe genetic disorders, and trials based on induction of muscle building to counteract the progressive wasting led to poor or transient success. A complete new look at therapy for MD The RegeneratioNfix project has taken a radical, ground-breaking approach to slow down MD. In previous research, the team showed that the transcription factor Nuclear Factor IX (Nfix) drives the switch from embryonic to foetal muscle formation – from slow to fast twitching and more mature muscle fibres. Prof. Graziella Messina, head researcher of the project, outlines the overall thrust and rationale of the project. ‘This project aimed to introduce a conceptually different proof of concept to preserve and maintain the musculature in dystrophic patients.’ Although this concept had already been demonstrated through Nfix silencing, all the evidence suggested that any other possible pharmacological approaches to reduce muscle regeneration and promote a slower twitching musculature may be equally effective. Assessing these research results, as Prof. Messina describes, ‘We proposed that a slower twitching and regenerating muscle might escape dystrophic muscle degeneration. Paradoxically, if muscles are forced to regenerate, this can exacerbate the phenotype.’ Testing the effects of Nfix The researchers crossed different MD animal models with mice lacking Nfix. Results showed an impressive improvement of the observable physical (phenotypic) and functional symptoms associated with dystrophic muscles in animals up to six months of age. ‘Importantly, this amelioration was also observed in the mdx Duchenne mouse model, thus demonstrating the broad validity of this approach.’ Prof. Messina points out. Lack of Nfix led to delayed and slow muscle regeneration upon acute injury and provoked a conversion of the musculature towards an oxidative metabolism, as published in Cell Reports. As for protection of the dystrophic muscle against further degeneration, absence of Nfix leads to a general switch towards a slow-twitching contraction and a reduced muscle regeneration, which better protect muscle wasting in time. These results are now in press in Nature Communications. Nfix modulation for future MD therapeutics Now just beyond project end, the RegeneratioNfix team are continuing to work on Nfix and its therapeutic use for MDs. The main focus is on the role of Nfix in macrophages, the most important players in tissue inflammation. During the project term, the researchers identified and characterised members of the pathway upstream from Nfix. Prof. Messina believes this will be the course of research that will result in possible pharmaceutical interventions to inhibit Nfix production. ‘In line with this, a “druggable” way to inhibit Nfix or its function will represent the future research topic of my laboratory.’ In parallel, they are developing more specific ways to inhibit Nfix using molecular design based on the crystalline structure of the transcription factor. Summing up the achievements of the project, ‘Both approaches will represent a translational outcome of the RegeneratioNfix project, which started as a fundamental research study and whose results might become a real opportunity to develop a potential therapy in human MD patients.’ Prof. Messina concludes.
Keywords
Muscle, muscular dystrophy, therapy, RegeneratioNfix, Nfix