Oral immunotherapy for cystic fibrosis
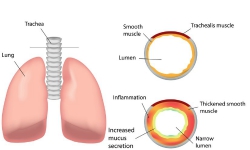
CF, caused by a mutation in the CFTR gene, leads to mucous accumulation in the lungs, predisposing individuals to infections, and ultimately, fatal pulmonary obstruction. More than 1 700 mutations have been identified with an overall prevalence of one in every 2 500 people. The vast majority of CF patients become infected with the gram negative bacterium Pseudomonas aeruginosa (PA). Antibiotic treatment is only effective in the initial stages of the disease; inevitable recurrent infections will culminate in chronic pulmonary infection. Intriguingly, PA strains from CF patients are more resistant to antibiotics than PA strains isolated from other patient groups. As CF is fatal and faces the growing threat of antibiotic resistance, it is of utmost importance to find effective alternatives to current antibiotics. Ten European countries collaborated to develop therapeutic avian antibodies IgY against PA with orphan drug designation. This was made possible through the EU-funded IMPACTT (Immunoglobulin IgY pseudomonas A clinical trial for cystic fibrosis treatment) project. Antibodies produced in the egg yolk Avian IgY antibodies were produced in the egg yolk of laying hens vaccinated against PA. The antibodies were purified from the egg yolk by water extraction, to produce a formulation containing only egg proteins and water. ‘When administered orally, the risk of serious adverse events should not be larger than the risk associated with eating eggs,’ explains IMPACTT project co-ordinator Prof Larsson. Previous phase I clinical studies have shown that if patients gargle with the IgY solution every evening, it prevents PA from entering the lungs. A single daily gargle was sufficient to prevent recurrent and chronic infections. The IMPACTT consortium wished to extend anti-Pseudomonas IgY antibody immunotherapy into a pharmaceutical treatment that will benefit the CF community within the EU and worldwide. Pre-clinical work by IMPACTT scientists indicated that oral administration of anti-Pseudomonas IgY is well tolerated and does not damage the gastrointestinal tract nor does it affect the normal and pathogenic bacterial microflora of treated mice. Mechanism of action studies revealed that IgY opsonises the bacteria and augments their internalisation into polymorphonuclear neutrophils. In turn, this implies a faster bacterial clearance in the CF airways and suggests that IgY antibodies could be used to boost innate immunity against PA. Furthermore, the consortium investigated over twenty strains of PA, including some of the antigens used for immunisation. ‘Anti-Pseudomonas IgY was shown to be immunoreactive against all of the tested strains strengthening its potential use as a prophylactic treatment against PA,’ Prof Larsson continues. Clinical trial The primary aim of the IMPACTT project was to conduct a randomised, placebo-controlled, double blind phase III clinical trial in various centres across Europe. 164 CF patients were recruited to investigate the critical preventive and therapeutic effects of anti-Pseudomonas IgY in CF patients. The trial assessed the recurrence of PA in the sputum of the patients who received oral anti-Pseudomonas IgY or a placebo formula for two years. In addition, it demonstrated that these antibodies were present in the oral cavity of patients for up to 24 hours without producing any adverse immune or allergic reaction. Chronic PA infection is the most common cause of morbidity and mortality in CF patients. The IMPACTT antibody-based intervention constitutes a valid prophylaxis that minimises the risk of antibiotic resistance. The findings of this study extend beyond CF, supporting the development of IgY-based oral immunotherapies to replace existing antimicrobials. A similar approach could also be used for the prevention of bacterial infections in animals.
Keywords
Cystic fibrosis, Pseudomonas aeruginosa, IMPACTT, IgY antibodies, phase III clinical trial