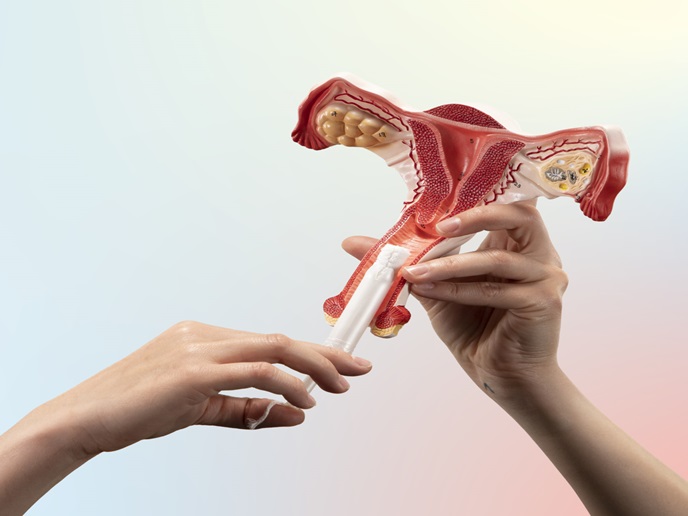
The tampon leading a women’s health revolution
For decades, invasive and uncomfortable screening methods in gynaecology have deterred women from seeking the preventive care they need. By using tampons for sample collection, the VMS project offers a way to disrupt the status quo. The approach makes health screenings more accessible, empowering women to take control of their health with comfort and confidence.
Tampons as tools of change
Traditional screening methods, such as speculum examinations and swabs, have remained largely unchanged for over a century, creating barriers to access. Many women and those assigned female at birth (AFAB) avoid screenings due to discomfort, embarrassment or logistical issues, leaving a significant proportion of gynaecological infections undiagnosed. “Over 30 % of women avoid HPV screenings,” says Valentina Milanova, VMS project coordinator and CEO of Tampon Innovations in Bulgaria, “while 70 % of infections are asymptomatic, increasing the risks for serious complications like cervical cancer and infertility.” The VMS project offers a groundbreaking alternative screening method: tampons repurposed as sample collection devices. This new approach increases accessibility and enables women to take charge of their health comfortably and privately. “We wanted to make preventive care something women can embrace, rather than avoid,” Milanova explains. VMS also aimed to bridge the gender gap in medical research by generating valuable data on the vaginal microbiome, a field historically underfunded and overlooked.
Validation and innovation
The journey to making VMS a reality involved several significant steps. Central to its success was clinical validation. Trials conducted in partnership with Liverpool Women’s Hospital and NHS Lothian in the United Kingdom demonstrated that tampons were as effective as swabs in detecting HPV and sexually transmitted infections. The project also introduced a digital platform that integrates with the at-home screening kits. Through the VMS app, users can access test results, consult with healthcare professionals and receive personalised treatment plans. This holistic approach represents a shift from traditional diagnostics to a more comprehensive model of care. Another highlight was the creation of a biobank for anonymised samples, aimed at advancing research into gynaecological conditions such as endometriosis and polycystic ovary syndrome (PCOS).
Tackling barriers head-on
Trying to shake things up in women’s and AFAB healthcare comes with a unique set of challenges. Navigating regulatory requirements required careful coordination with legal experts. VMS also secured funding through a combination of public grants and private investments. Developing the technology was equally demanding. The team collaborated with academic partners to refine a proprietary DNA extraction method for tampons, while overcoming supply chain disruptions by diversifying suppliers and investing in in-house manufacturing capabilities. VMS has received widespread praise from users and healthcare providers alike. Over half of the service’s users were first-time participants in gynaecological screening programmes, and 78 % of them reported that the at-home kits eliminated barriers such as embarrassment and logistical difficulties. Crucially, healthcare providers see immense value in the initiative, which reduces waiting times and enhances patient outcomes. Public health collaborations (including partnerships with United Kingdom NHS Trusts) have expanded its reach.
The future of VMS
Long-term plans for the project include expansion into North America, introducing diagnostic tools for fertility assessments, and working with global health organisations such as the World Health Organization (WHO). “Project VMS is more than just a screening tool, it’s a new way of thinking about gynaecological health,” Milanova adds. “We want every woman and person AFAB, regardless of location or socio-economic status, to have access to care.”
Keywords
VMS – Vaginal Microbiome Screening, VMS, Bulgaria, Vaginal Microbiome Screening, healthcare, gynaecological, screening, tampon