Novel biodegradable bandage controls severe bleeding and leakage of body fluids during surgery
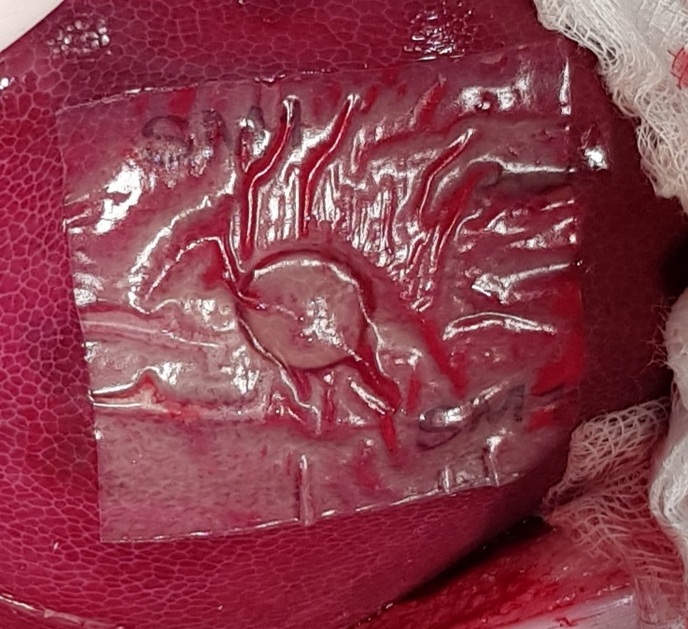
Standard surgical methods such as sutures and ligatures have been used for decades to prevent and stop bleeding. In many cases, such techniques have proven ineffective or impractical when dealing with severe haemorrhaging. The EU-funded sFilm-FS project developed a product composed of a biocompatible, bioabsorbable polymeric film with a biological glue known as fibrin sealant. Also named sFilm-FS, this human body-friendly bandage is used to prevent blood and body fluid leakage in internal organs. “The product is much more effective, faster and easier to use than any other standard of care,” comments project coordinator Orgad Laub. It is absorbed by the body, allowing full and effective healing of the injured tissue. Faster recovery time means a shorter hospital stay and less burdened health system. sFilm-FS is utilised when haemostasis – a process to prevent and stop bleeding – isn’t effective for soft tissue bleeding. It is also intended for use during sealing in gastrointestinal surgery. “Unlike open surgery, for minimally invasive surgery treatment there are very limited tools to handle bleeding scenarios,” explains Laub. “For sealing, such as gastrointestinal sealing, there is currently no registered device, so sFilm-FS will be a novel solution.”
Unparalleled strength and sealing ability
The fibrin sealant attaches and fixes the polymeric film to the damaged tissue. The film seals the tissue, similar to a rubber patch that seals an air leak in a flat tyre. In the case of bleeding, the fibrin sealant accelerates the coagulation of the blood under the film. Unlike other products that contain similar components, sFilm-FS is based on the sealing capacity of the polymeric film. Project partners successfully validated sFilm-FS by testing it on rats and pigs. Severe bleeding was stopped within 2 minutes in rat and pig spleen and liver punctures. sFilm-FS also successfully sealed gastrointestinal injuries. Toxicity tests demonstrated that sFilm-FS was non-toxic, even when using maximum doses, and all treated animals recovered without any side effects. EU regulatory agencies in Austria and Slovenia evaluated and approved the product for human clinical studies. Human trials started in September 2020.
Step change in operating rooms
Feedback provided by the United States Food and Drug Administration led to the development of a unique terminal sterilisation step that considerably improves the safety of sFilm-FS and slashes manufacturing costs. Thanks to the thin film that contains the fibrin sealant, the product could also be developed for minimally invasive surgery treatment. The project team is working on a laparoscopic tool that will extend the use of sFilm-FS to all surgical procedures. “sFilm-FS is a novel surgical device that will help control severe and moderate bleeding and tissue sealing during both open surgery and minimally invasive surgery,” concludes Laub. “These are considered unmet needs that will significantly shorten surgery and healing times.” Ultimately, the project should improve safety and lessen the risk of post-operative complications while reducing mortality rates.
Keywords
sFilm-FS, bleeding, surgery, tissue, fibrin sealant, polymeric film, bandage