Scientists find on-off switch for blood-vessel growth
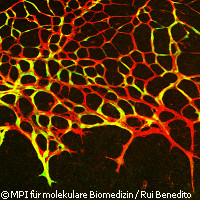
European researchers have identified an 'on-off' switch for blood vessel growth. The findings, published in the journal Cell, could aid in the development of treatments for diseases and conditions characterised by either too much or too little blood vessel growth. During development, growth and healing, new blood vessels form an intricate network, branching out where necessary to ensure that all tissues are supplied with a steady flow of oxygen and nutrients. When the process goes wrong, the results can be disastrous; heart attacks occur when the blood supply to the heart tissue is blocked. In this case, scientists would like to know how to encourage the growth of new blood vessels in the heart. On the other hand, cancerous tumours are kept alive by a network of blood vessels; here, blocking the growth of new blood vessels would effectively starve the tumour. Scientists have long sought the mechanism which determines when a blood vessel should branch in two and when not. In this latest study, German and UK scientists identified a molecular switch that controls the fate of individual cells. The switch in question involves a protein called Notch, which is found on the surface of the cells lining the blood vessel walls. Different proteins can attach themselves to Notch; some switch it on, and some switch it off. If Notch is switched on, the cell becomes sensitive to a molecule called Vascular Endothelial Growth Factor (VEGF). As its name suggests, VEGF promotes the growth of new blood vessels. In this study, the researchers found that a protein called 'Jagged1' switches Notch on, and so causes new blood vessels to be formed. Jagged1 sits on the surface of cells, and so is able to attach itself to the Notch switches of neighbouring cells. 'For the first time, we now understand how these individual components work together,' commented Professor Ralf Adams of the Max Planck Institute for Molecular Biomedicine in Germany. 'In experiments on mice, we want to learn how to actively control the growth of blood vessels.' Ultimately, medicines could one day be used to do the same in people, he added. Currently, some cancers and eye conditions are treated by blocking the activity of VEGF. However, these treatments are expensive and the side effects make them inappropriate for many patients. 'By clarifying the function of Jagged1, we hope we have found a real alternative for future therapies,' said Dr Rui Benedito, also of the Max Planck Institute for Molecular Biomedicine. The next step for the researchers is to thoroughly investigate both the effectiveness of treatments interfering with Notch and whether or not they cause dangerous side effects. This is an important question because Notch has important functions in the development of the nervous and immune systems, for example. 'Notch, Dll4 [a protein known to slow the formation of new blood vessels] and Jagged1 have important tasks in other organs and cell types,' explained Professor Adams and Dr Benedito. 'This makes limiting the effects of [a drug] to the blood vessel cells challenging. We hope, however, that our work will lead to the development of new medicines.'
Countries
Germany, United Kingdom