Retinoic acid/arsenic-based APL therapy now fully deciphered
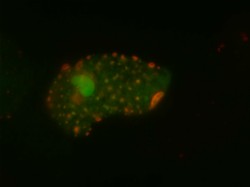
Prior to the project, Prof. de Thé and his team had already demonstrated how RA and arsenic directly bind PML/RARA — the driving oncoprotein in APL — and promote its degradation. With the STEMAPL (APL a model for oncogene-targeted leukaemia cure) project, they went a step further: they showed how this therapy-induced degradation allows for the re-assembly of distinct nuclear domains called PML nuclear bodies. ‘PML nuclear bodies act as post-translational modification factories. They participate in stress response,’ Prof. de Thé explains. ‘Using mice models, we demonstrated that, in APL, the PML nuclear body reformation induced by RA and arsenic treatment activates a senescence programme that includes the activation of the P53 tumour suppressor. Interestingly, PML are known markers of senescent cells. This RA- or arsenic-initiated PML/P53 checkpoint is absolutely required for the definitive clearance of the disease.’ Another key achievement of the project is the demonstration of mutations’ existence in PML (and not only in PML/RARA, as initially expected) in therapy-resistant patients. As Prof. de Thé points out, this is the first-ever example of a situation in which the resistance mutations are on the anti-oncogene, rather than its oncogenic counterpart. This enabled the team to fully validate in patients the physio-pathological model initially established in mice, which Prof. de Thé considers as ‘a remarkable example of the predictive power of mouse models to understand and optimise therapy.’ From APL to other types of leukaemia The information gathered under STEMAPL is of high value to all cancer researchers, potentially beyond the sole case of APL. ‘There are not many examples where you can cure cancer knowing how this is achieved. APL is a unique system for exploring the downstream targets of therapy, and it yielded the unexpected implication of PML. Knowing the basis of response, one can then try to transpose it to other conditions. While clearly this is not the universal pathway to cancer cure, it is possible that it is shared between more than one leukaemia,’ Prof. de Thé says. Whilst there is still a long way ahead, the team strongly believes that their findings will not be limited to APL. In fact, other types of acute myeloid leukaemia (AML) were already found to present abnormal PML nuclear body formation. Now that STEMAPL has come to an end, Prof. de Thé will be applying for a new ERC grant focusing on the anti-cancer properties of PML, innovative clinical trials, and other conditions with a focus on the activation of PML. ‘There is good clinical evidence that some progress may be expected,’ he enthuses. In the meantime, the now largely understood RA/arsenic combination has now become the gold standard all over the world. It cures over 95 % of treated patients without resorting to DNA-damaging therapy, and provides a unique example of success for targeted therapy. ‘This success demonstrates that oncoprotein degradation is a feasible therapeutic goal, and it highlights one of the rare conditions where the biochemical and cellular activities of an anticancer therapy are understood in significant details,’ Prof. de Thé concludes.
Keywords
STEMAPL, Hugues de Thé, leukaemia, APL, acute promyelocytic leukaemia, retinoic acid, arsenic, tumour, oncoprotein