Innovative platform helps acute care patients communicate
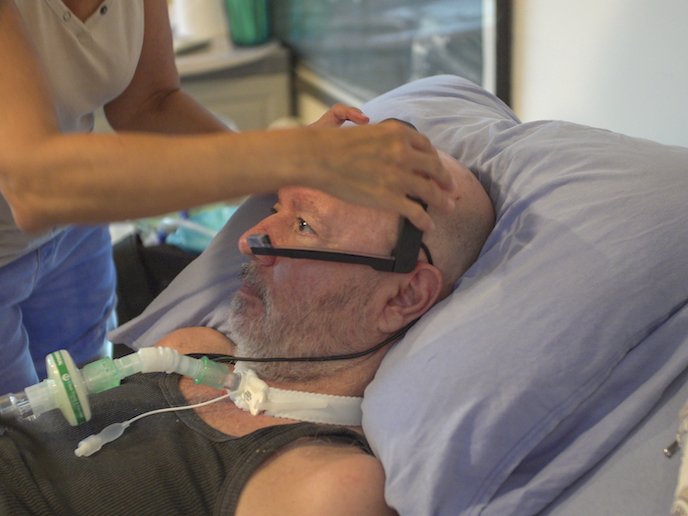
Communication is practically impossible for patients, often on ventilators, in intensive care units (ICUs). The resulting isolation and disorientation can lead to anxiety, cognitive decline and even delirium. “Most communication solutions based on tracking eye movements tend to be cumbersome, screen-dependent and are usually not available 24/7. They also rely on a bedside presence to interpret communication, with training taking up to a week; problematic as the average ICU stay is 3.8 days,” says Or Retzkin CEO and co-founder of EyeControl, the project host. In response, the EyeControl team developed a wearable, screenless communication device and platform, EyeControl-Med, with an average training time of under 20 minutes. “Aside from its cutting-edge communication features, which increase patient engagement and emotional wellbeing, it also has monitoring functions,” summarises Retzkin. The EU’s EIC Accelerator programme supported the team’s R&D, clinical trials and regulatory milestones, taking their EyeControl-Med device from prototype to a commercial product, including multiple patents. As well as ICUs, the device is targeted at long-term acute care facilities and rehabilitation centres and has already secured significant publicity, including winning the Genesis Prize Start-Up Nation Central’s COVID-19 Innovations competition.
The digital personal assistant
The EyeControl-Med platform comprises two main elements. A headset with a bone conduction component supplies audio to the patient. Meanwhile a head-mounted camera tracks eye movements, sending this information to a small processing unit that uses machine learning to analyse and translate gestures into real-time communication, transmitted through a speaker. This all takes place without the patient having to use a screen. In parallel, this data is relayed to a ‘virtual nurse dashboard’, enabling patients to be remotely monitored and care staff to control communication sent to the patient. This includes messages to keep patients orientated such as date, time and a reminder of where they are. Additionally, the family can record voice greetings through a telephone-based app. Data security and privacy is assured with the use of encryption techniques throughout, as well as compliance with GDPR and ISO 27001 and 27799.
Clinical studies
A phase I feasibility trial was conducted at Israel’s Beilinson Hospital in 2021, where nearly half the participants had COVID-19, many isolated from loved ones. “This resulted in positive preliminary usability data, alongside testimonials from families, medical teams and crucially, patients, one of whom said: ‘The process gave me a feeling of control in my state of helplessness, and that motivated me, and cheered me up’,” recalls Retzkin. This has led to additional clinical activity in US and Israeli hospitals, including an extended phase II comparison trial at Beilinson to demonstrate clinical benefits.
Rolling it out
According to Retzkin, EyeControl’s communication platform contributes to three global healthcare trends. Firstly, brain-tech, which maintains patient cognition in critical care settings to improve outcomes and quality of life post-discharge. Secondly, value-based care which humanises healthcare. And lastly, a focus on patient communication and environmental control in acute care, especially post-COVID. “COVID-19 has heightened awareness of the communication needs of critical care patients, so our vision is that every ICU patient will receive EyeControl-Med upon admission,” concludes Retzkin. EyeControl-Med is commercially available to Clalit health service hospitals in Israel, is FDA listed in the US and CE marked in Europe. The team is in discussion with the procurement departments of Israeli, US and UK hospital ICUs. An outpatient version of the product, EyeControl-Home, can already be purchased privately, eligible for state reimbursement in Israel and the UK.
Keywords
Eyecontrol, eye movements, ICU, COVID-19, communication, patient, acute care, brain-tech, wearable