Innovative gold-based drugs for guided anticancer therapy
The therapeutic potential of metal-based compounds is not new and dates back to ancient Greece and China. Platinum-based drugs, such as cisplatin and its derivatives, are used as anticancer drugs; however, there is a growing demand for alternative metal-based compounds with enhanced cytotoxicity and pharmacokinetics and reduced side effects.
Novel gold-based compounds
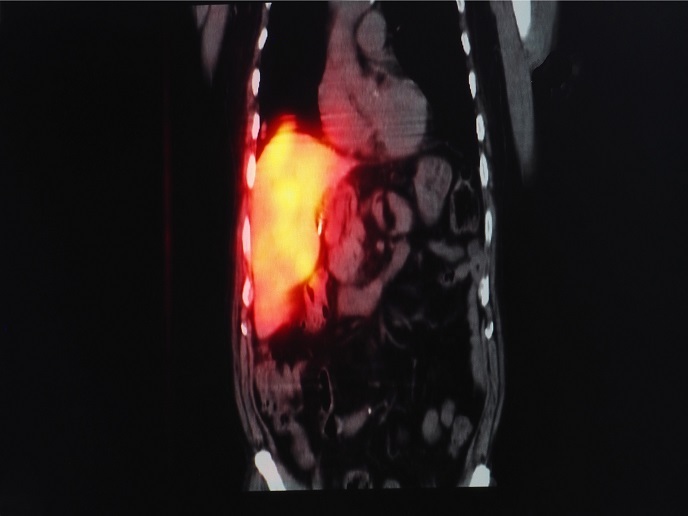
Undertaken with the support of the Marie Skłodowska-Curie Actions programme, the PhoRAu project developed compounds based on gold(III) decorated with radioisotopes. Researchers wanted to understand when and how gold(III) prodrugs are converted to gold(I) drugs. “Indeed, this promising class of molecules can result in novel cytotoxicity mechanisms, as they can potentially undergo controlled reduction into gold(I) compounds in cell compartments,” explains the Marie Skłodowska-Curie research fellow Alessio Terenzi. Researchers wanted to combine therapy with imaging capabilities, so they labelled the gold prodrugs with radioactive iodide. This allowed monitoring of the biodistribution of the prodrug in vivo via positron emission tomography (PET), similarly to the radioactive fluoride labelled glucose used for imaging tumours in oncology. Unlike traditional strategies where radionuclides are covalently attached to organic molecules, PhoRAu scientists used inorganic reactivity to perform the labelling. Importantly, this straightforward inorganic reaction between the radioactive iodide and gold(I) led to the formation of the desired gold(III) prodrug that they wished to produce. They also investigated the thermodynamic, kinetic and redox properties of these gold(III) complexes in aqueous solution and in the presence of biological molecules. Biodistribution studies of the gold(III) prodrugs allowed scientists to monitor the gold reduction process in vivo and understand how gold(III) complexes are metabolised and activated. Following PET imaging, the gold(III) prodrug accumulated in the major organs, such as lungs, kidneys and liver, through the blood circulation. Intriguingly, the gold(III) prodrug was not immediately reduced to its gold(I) analogue, but reached some organs in its oxidised form, providing important insight into gold-based therapeutics.
Project dynamics
Through their radioactive gold derivatives, PhoRAu researchers have managed to add an imaging functionality to their candidate gold-based prodrugs and guide therapy through PET imaging. “Our strategy overcomes an intrinsic limitation of metal-based drugs which can rarely be monitored in real time in vivo,” says Terenzi. Significantly, the method is based on an original labelling concept that can find additional applications for the tagging of other anticancermetal-based drugs. Furthermore, the data on the interplay between gold(III) and gold(I) species in vitro and in vivo, shed light on the mechanism of action of this family of anticancer drug candidates. In vivo biodistribution studies of gold complexes are still quite rare and therefore the PhoRAu work is seminal in the field. Although the clinical application of these candidate compounds is still years away, Terenzi notes: “Our approach will inspire others and lead to the development of drugs that can ultimately reach clinical trials.”
Keywords
PhoRAu, gold, metal-based drugs, cancer, radioactive iodide, labelling, PET imaging, photodynamic therapy