Disruptive diagnostic tool discriminates between viral and bacterial infection
Globally, an estimated 3 billion patients seek medical attention for suspected acute infection each year. Medical history, physical findings and other ancillary medical tests often do not provide definitive discrimination. Misdiagnosis of disease aetiology may alter the trajectory of patient care, resulting in unnecessary diagnostic tests or prescription of antimicrobials.
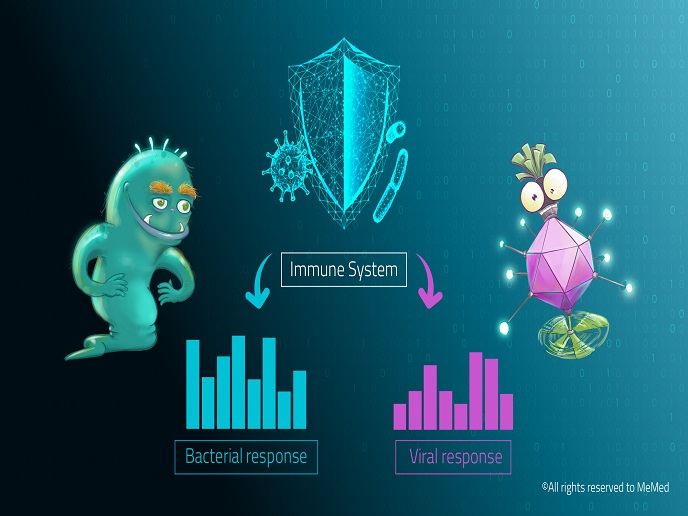
Host signature differentiates between bacterial and viral infection
Researchers in the EU-funded AutoPilot-Dx project developed a novel ELISA-based assay that accurately distinguishes between bacterial and viral infections. ImmunoXpert™ measures the circulating levels of three host proteins, tumour necrosis factor-related apoptosis-inducing ligand (TRAIL), interferon gamma-induced protein-10 (IP-10) and C-reactive protein (CRP). “These proteins exhibit distinctive expression and complementary dynamics in host responses against bacterial versus viral infection,″ explains VP Scientific Affairs of MeMed, Tanya Gottlieb. The signature based on these three host proteins was discovered and validated previously by the consortium in the large-scale prospective study ‘Curiosity’ with over 1 000 adult and paediatric patient infections. This signature, named BV™, displayed 92 % sensitivity and 89 % specificity across multiple pathogens, and superiority to biomarkers in routine use. It was further validated in two double-blinded external clinical studies enrolling over 1 300 children. BV™ can be measured using the ImmunoXpert™ immunoassay manually or through the help of a robotic platform. The test result is a score between 0 and 100, with low scores indicative of viral infection and high scores of bacterial infection.
Clinical impact of the BV™ immune signature
Fever accounts for 10-25 % of paediatric visits to the emergency department and as many as 20 % of these are due to an unidentifiable source. Diagnostic uncertainty is reflected by the variable increase in antibiotic prescription worldwide, leading to fundamental individual and global health consequences, including the rise of antimicrobial resistance. BV™ is uniquely positioned to improve the management of paediatric patients with respiratory tract infections and fever without source that account for roughly 50 % of doctor’s visits. To estimate the clinical utility of BV™, researchers calculated the potential of the assay to reduce unnecessary antibiotic use as 87 % in children and 91 % in adults. “BV™ represents an innovative and actionable tool that will help clinicians make better informed management decisions for patients with suspected acute infection, helping to fight antibiotic overuse without compromising patient safety,″ emphasises Gottlieb. The European market for ImmunoXpert™ diagnosis is estimated at over EUR 1 billion, with market entry points being the emergency department and paediatric hospital wards. AutoPilot-Dx partners are also in the process of developing a point-of-care platform called Key™ that will enable the BV™ test to be performed when and where needed, and provide a result within 15 minutes. The launch has been scheduled for 2020 in Europe and then in the United States. “The vision of our company is to decode the signals of the host response into simple insights that improve people's lives,″ concludes Gottlieb. Project partners are currently working on innovative host response-based diagnostics to help manage sepsis and plan to leverage the measuring capability of Key™ to bring novel tests to the market.
Keywords
AutoPilot-Dx, viral infection, bacterial infection, ImmunoXpert™, BV™, tumour necrosis factor-related apoptosis-inducing ligand (TRAIL), interferon gamma-induced protein-10 (IP-10), C-reactive protein (CRP)