Repurposed drug offers hope for those recovering from stroke
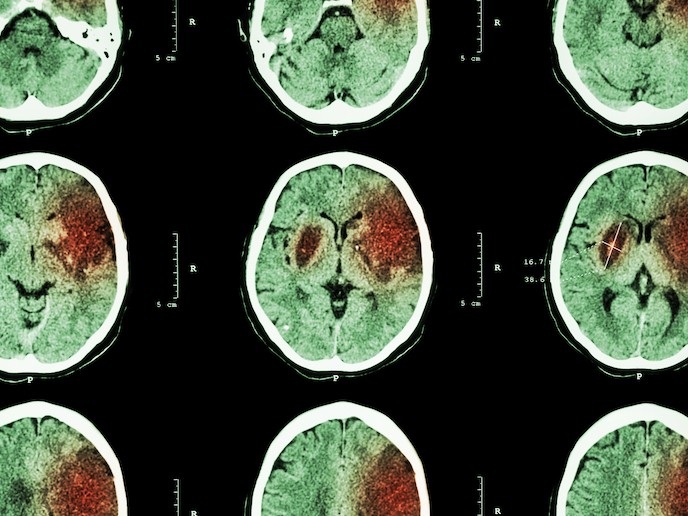
Stroke is caused either by haemorrhage (bleeding due to rupture of blood vessels in the brain) or by a blockage of arteries in the brain. It is estimated to affect 13.7 million people worldwide every year, with 116 million healthy years lost (Disability-adjusted life years – DALYs) due to death and life-long sensory, motor or cognitive disabilities. Over the last two decades, developments in prevention and treatment have reduced the proportion of people suffering from a stroke, while recovery has also significantly improved. Despite this, with an increase in the number of over 70-year-olds, the absolute number of strokes is due to increase. With one report anticipating an increase of 34 % in stroke incidents between 2015 and 2035, prevention and treatment are fast becoming high clinical priorities. Within the first hours after a stroke, patients can be treated with recanalisation therapies, such as thrombolysis, but globally only a minority of patients actually receive this treatment. Later, during stroke recovery, patients may also receive rehabilitation therapies but these are often insufficient and result in limited effect. There are currently no medical treatments available on the market to support rehabilitation. With EU support, under the SINSTRO project, Sinntaxis plan to repurpose the drug SIN020 for stroke recovery. As it was previously under clinical development for another illness, it can be entered straight into a Phase II clinical trial.
SIN020 – the repurposed drug
SIN020 is an inhibitor of the metabotropic glutamate receptor known as mGluR5, and, as a key ingredient for neuro-transmitters, can modulate the brain’s response to a stroke. SIN020 has been shown to work in animal models, where it has enhanced the brain’s capacity to recover from the stroke and reverse lost functions. Treating mice and rats, subjected to experimental stroke, with an experimental mGluR5 inhibitor, tests showed that starting treatment 2 or 10 days after stroke significantly improved limb function. Additionally, this effect persisted even after treatment was discontinued. “Transferred to the clinical situation, this treatment could start well beyond the 4.5 hours’ therapeutic time window of thrombolysis, meaning that essentially all eligible stroke patients could be treated with the drug,” explains lead researcher Tadeusz Wieloch.
Addressing a major unmet medical need
Successful development of SIN020 could enhance brain function after a stroke, resulting in increased quality of life for stroke patients. It will also decrease the welfare cost of stroke, estimated at EUR 64 billion in 2010. “What is especially exciting is that: as this is a repositioned drug candidate, a lot of the groundwork has already been done. This shortens the development time needed, meaning we can get it to patients faster,” says project coordinator Tomas Eriksson. Sinntaxis is currently planning for a clinical proof of concept study with the aim of starting within 12-18 months.
Keywords
SINSTRO, stroke, drug, welfare, brain function, thrombolysis, therapeutic, recanalisation therapies, rehabilitation, mGluR5 inhibitor, patient