First ever gene therapy trial for blindness gets underway
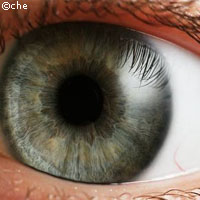
The world's first clinical trial to treat childhood blindness with gene therapy has got underway in the UK. The trial, which was partly funded by the EU, involves adults and children who have an inherited retinal disease called Leber's congenital amaurosis (LCA). The disease is caused by a mutation in a gene called RPE65, which controls the production of an enzyme responsible for recycling retinol, a chemical which is needed to capture light. If the retinol is not recycled correctly, the eye's light-sensitive cells run out of supplies and stop working. Patients with the condition suffer from severely impaired vision from a very young age. Currently, there is no effective treatment available. 'We have been developing gene therapy for eye disease for almost 15 years but until now we have been evaluating the technology only in the laboratory,' said Professor Robin Ali, who is leading the team conducting the trial. 'Testing it for the first time in patients is very important and exciting, and represents a huge step towards establishing gene therapy for the treatment of many different eye conditions.' In the new treatment, surgeons deliver healthy versions of the gene to the retina, where they are carried into the cells by a specially designed harmless virus known as a vector. Once there, the healthy genes should start producing the retinol recycling enzyme correctly, and restoring the function of the light-sensitive cells. The team conducting the trial includes researchers from Moorfields Eye Hospital and University College London's Institute of Ophthalmology. Early trials of the treatment in dogs have shown that the therapy is effective at both improving and preserving vision. The current trial is designed to test how safe and effective the treatment is in humans. The first operations have already been carried out on young adults who developed the condition as children, but the researchers warn that it will be some time before the results are known. In the long term, the researchers expect the best outcome in younger patients, as they will be treated when the disease is still in its early stages. 'Some indications of the results of the trial may be available within several months. However, subjects will need to be followed up to assess the long term effects of the treatment,' commented retinal specialist Professor Tony Moore, who is involved in the trial. 'It will be many months before we have the full picture.'
Countries
United Kingdom