Customised minimally invasive medical devices
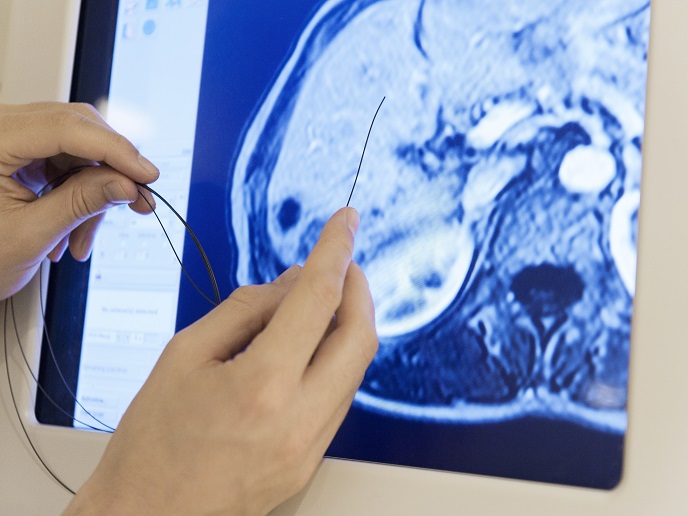
Easy and reliable navigation of instruments is key for successful minimally invasive surgery. Currently, surgeons have to choose from a predefined limited spectrum of devices available from medical device manufacturers. Customisation of a disruptive innovation The OPENMIND project approach involves the development of a highly flexible process chain for the on-demand production of entirely customised minimally invasive medical devices. This goal comes along with a technological and economical breakthrough for medical device manufacturing companies. “Development of this innovative production system enables continuous and cost-efficient production with lot sizes down to an individual product,” explains Jonathan von Helden, researcher at Fraunhofer IPT, the project coordinator. The researchers linked together various software, hardware and metrology modules in a continuous and adaptive production system. “At the same time a technology readiness level of TRL6 was reached for the overall process chain – a great achievement for OPENMIND,” notes von Helden. In the context of customisation, online monitoring tools such as a database, a process model and predictive algorithms are of particular importance. Not only do they ensure a high product quality, they also provide and predict the parameter sets for the production of individualised guidewires. Synchronisation During the final implementation and commissioning phase, synchronisation of different production systems developed at the laboratories of each individual partner turned out to be the key challenge. As joint project leader Jonas Dorissen explains: “Many of those systems represent special purpose machinery. Very rudimentary parameters like the process speed or material conveying speed needed to be harmonised to ensure continuous production.” Close cooperation of all project participants was the solution and permanent controlling was carried out. Future commercialisation Aided by EU funding, TRL6 was successfully achieved – i.e. prototype manufacture in an operational environment. Other individual TRLs have also been clocked up. “The pullwinding process including new developed online process monitoring has a technical readiness of TRL7 and can be transferred to TRL9 in due course after the project with some industrialisation effort,” explains von Helden. An industrial application for individualised guidewires seems feasible with OPENMIND technology if some technological challenges, such as laser structuring of micro-profiles, are overcome. The consortium partner Nano4Imaging, as a potential end user of the project’s outcome, will work on developing the market interest by promoting the new technology. Some partners will keep on working to improve their respective modules, possibly by means of a future follow-up project. Fibre-reinforced plastics and MRI-guided surgery Development of new compatible devices and advancements in MRI technology will be the next revolution in the field of minimally invasive surgery. This is set to lead to development of completely new procedures grouped under the term interventional MRI, or iMRI. So far, the micro pullwinding technology developed at Fraunhofer IPT has been used to manufacture multifunctional puncture needles, different types of guidewires and catheters, as well as aneurysm clips. As von Helden emphasises: “The particular characteristics all these minimally invasive medical devices have in common are the use of fibre-reinforced plastics without any macroscopic metallic components. These devices therefore have great applicability for MRI-guided surgeries.”
Keywords
OPENMIND, minimally invasive, medical device, MRI, surgery, fibre-reinforced plastics, interventional MRI, pullwinding technology