Breakthrough neuromodulation technology for epilepsy treatment
Epilepsy is a major brain disorder affecting over 50 million people worldwide. Patients experience repeated seizures, literally ‘electrical storms’ of abnormal activation of nerve cells, which cause significant harm and negatively impact their quality of life. Pharmaceutical compounds are ineffective in 30-40 % of epilepsy cases, necessitating alternative approaches with minimal side effects. Various technologies have surfaced over the years that involve delivering tiny electrical currents to the brain or manipulating nerve cells by light, magnetic or ultrasound fields. These technologies resemble pacemaker devices for brain disease treatment. While these do not require systemic drug administration, they involve invasive interventions and genetic modifications whose long-term effects are unknown.
Breakthrough modulation of ion levels in neurons
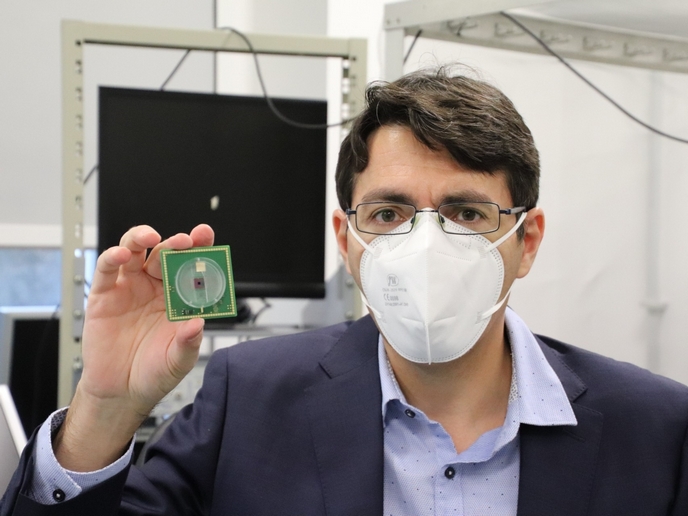
The EU-funded IN-FET project set out to revolutionise epileptic seizure treatment by acting on the underlying principle of brain electrical activity: ions. The movement of ions through neuronal membranes lies at the heart of any brain activity, causing neuronal firing, synaptic transmission and neuronal communication. IN-FET explored the visionary idea of neuromodulation through ionic actuation at cell level. “The idea was to regulate abnormal electrical activity in nerve cells by altering the local concentration of key ions such as potassium and calcium, crucial for neuronal activity,” explains project coordinator Michele Giugliano. The consortium developed a device that works by using special materials and tiny sensors to control and monitor the activity of nerve cells in the brain. The device consists of electro-activated polymers that can be loaded with specific ions and then released in the extracellular environment around neurons, to alter how they behave when they receive an electrical signal. These polymers are integrated with ultra-sensitive nanowire transistors capable of detecting the electrical activity of individual nerve cells. The transistors penetrate membranes and detect cell electrical activity at unprecedented spatial and temporal resolutions. The core technological breakthrough of IN-FET is that the device can continuously adjust itself through a closed loop regulation mechanism. If the sensors detect too much activity – during a seizure for example – the device can release ions to calm the cells down. If there’s too little activity, it can capture ions to help the cells become more active. This way, the device can regulate nerve cell activity in a precise and controlled manner without the need for drugs or invasive surgery.
Validation and potential applications
Although more work is required to assess the compatibility of the IN-FET system in invasive implants in humans, the consortium has successfully demonstrated in vitro its ability to modulate the extracellular concentration of potassium ions in neuronal networks. The team are considering international collaboration for optimising system performance for animal testing in the near future. As Giugliano points out: “The concept of ionic actuation is not limited to epilepsy treatment. Every excitability disorder or neurological disease where electrical stimulation and brain pacemakers are used could benefit from our technology.” The IN-FET system could be adopted in high-frequency deep-brain stimulation applications, such as those employed in the treatment of Parkinson’s disease. Moreover, it could be used in the field of neuroprosthetics for the development of more efficient brain-machine interfaces in central and peripheral nervous systems implants. Finally, new routes for the commercialisation of the current IN-FET technology are under consideration as they could be very useful in high-throughput platforms for drug screening and discovery.
Keywords
IN-FET, ions, epilepsy, brain, seizure, neuromodulation, electrical activity, transistors, electro-activated polymers