Exploring the molecular machinery of kidney cell gateways
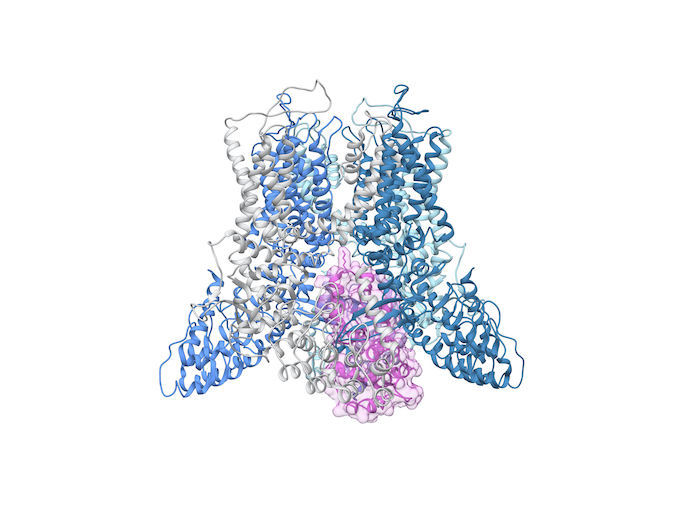
Discovered by the Physiology department of Radboud University in 1999, the TRPV5 (transient receptor potential vanilloid channel) specifically allows the passage of calcium. This mineral is vital for healthy bones and teeth as well as preventing osteoporosis, among other functions. But it was not known how this calcium channel exactly works. What does the molecular machine that ensures the gate opens and closes look like? “Our main goal was to deliver detailed molecular insight into this calcium channel TRPV5,” says says Jenny van der Wijst, coordinator of the CRYO-EM TRPV5 project which was hosted by SKU – Radboud University Nijmegen.
Creating a 3D protein map
“Until recently, it was difficult to visualise the atomic structure of large membrane proteins like TRPV5. So we turned to the cryo-electron microscope, or cryo-EM for short, a revolutionary technique awarded the Nobel Prize in 2017,” explains van der Wijst. The EU-supported project used TRPV5 protein purified from mammalian cells and reconstituted in lipid nanodiscs, small disc-like lipid bilayers surrounded by a membrane scaffolding protein. These proteins were added in large quantities to water, where they could move freely. Van der Wijst worked closely with Yifan Cheng, from the University of California, whose expertise in structural biology dovetailed with van der Wijst’s biophysical background in ion channel functioning. “We froze the samples and, using this state-of-the-art microscope, took 2D photos of the proteins from all sorts of angles. Using chimera-specialised software for interactive visualisation and analysis of molecular structures, we constructed a 3D image of TRPV5 from the thousands of 2D pictures,” remarks van der Wijst, who received support from the Marie Skłodowska-Curie Actions programme.
Therapeutics on the horizon
“We produced several high-resolution structures of TRPV5. These could enhance understanding of the pathophysiology, in other words the effects of disease on body function, of various human diseases,” she adds. This detailed structural analysis may demonstrate how a gene mutation leads to impaired function: “Specific for TRPV5, we know several genetic variants that are linked to kidney stone disease.” Knowledge gained from high-resolution structures could ultimately support drug development programmes, as they may provide insight into the actions of pharmacological compounds. Van der Wijst adds that TRP channels are linked to various pathologic conditions impacting on the kidney and intestines. “So it’s important to fully understand how molecular components, like natural or non-natural substances and lipids, affect the ion channels.” Though the project has ended, it has sparked new research funded by the Dutch Research Council. “Besides obtaining a clear idea of the structure of the ion channel, we also visualised its blocking by an associated protein called calmodulin." “It’s a mystery how this controls calcium flow through TRPV5, though we see it closes the gateway like a cork in a bottle of wine,” concludes van der Wijst. The next step is to shed new light on this on-off mechanism in the TRPV5 channel.
Keywords
CRYO-EM TRPV5, calcium, calmodulin, cryo-electron microscope, ion channels, kidney, membrane proteins, nanodiscs, pathophysiology, TRPV5