Protein molecules 'addressed' for delivery to cell membrane
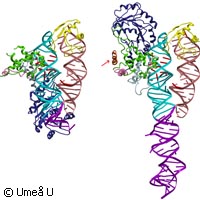
In a new study published in the journal Nature Structural and Molecular Biology, researchers at Umeå University in Sweden show how a cell ensures the correct distribution of proteins within its interior. What they created was a detailed picture of proteins furnished with a kind of label that serves to guide the proteins through the cell membrane. Most organisms with a complex structure are made up of countless cells, which in turn are also full of complex forms. Cells achieve such sophisticated organisation in large part by ensuring that their various proteins end up in the right place. How they manage this feat is of great interest to scientists. If we can understand how cells determine which protein goes where inside a cell, and which are exported, we may then be able to redesign the system to our advantage. However, the mechanism whereby distinct families of proteins are dispatched to their proper place in the cell remains one of the most intriguing questions in biology. Even the first step is mysterious: the apparently bizarre process of large polypeptides through the dense, impermeable interior of the endoplasmatic reticulum membrane. Nevertheless, scientists recognise some of the basic principles of the process; one major insight, established recently by the Umeå researchers, explains how proteins are exported even entirely from the cell. The first step of the export machinery relies on a molecular complex. This is made up of a small molecule of ribonucleic acid (RNA) intertwined with five specific polypeptides. Together, these constitute a signal recognition particle (SRP). This complex seeks out and combines with those proteins destined for export. Like SRP, one additional principle essentially found in the same form in all cells has already been discovered: the 'address label'. Cells seem to use short sequences of amino acids that make up a protein as labels for the protein's final destination. Without this signal sequence, a protein cannot interact with the SRP and move across the endoplasmic reticulum. This particular signal is removed in the process of moving across the membrane, and then another signal comes into play. Other sequences of amino acids may also act as a signal for further sorting steps; this is an exciting area of research. One of these additional peptide labels is known to scientists; anchor sequences. The anchor sequences are often found at the end of proteins. Composed of hydrophobic amino acids, they act to fix proteins tightly to membranes. Strangely, some anchor sequences function only in their ultimate location, which is outside the endoplasmic reticulum membrane or any other membrane in the export pathway. These complicated mechanisms are at work in all animal and plant cells, but the methanococcus jannaschii - an autotropic hyperthermophillic organism that belongs to the kingdom of Archaea - is a lot simpler. This single-cell microorganism offered the Umeå scientists a more straightforward model system in which to study the SRP structure with and without the guiding signal sequence. The technology they used is called X-ray crystallography. 'The structural changes were considerably greater than what was previously predicted,' explains Elisabeth Sauer-Eriksson, a professor in the Department of Chemistry at Umeå. 'They provide us with detailed explanations of what role SRPs play in protein transport. These structural specifications can also serve as a model of how SRPs function at various levels during protein transport.' If the ingenious mechanisms through which cells label and sort their proteins for export could be unravelled, genetic engineers could then make microorgamisms secrete any protein they like. The study was funded by the Swedish Research Council, the Umeå Centre of Microbial Research and the Kempe Foundation.For more information, please visit: Umeå Universityhttp://www.umu.se/english/Nature Structural and Molecular Biologyhttp://www.nature.com/nsmb/index.html