Scientists solve mystery of how old cells become new
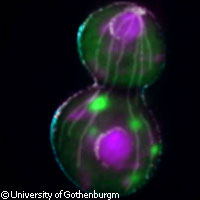
Researchers have discovered how old and damaged mother cells are able to produce healthy new daughter cells, a process which has been a mystery to scientists until now. The ground-breaking research was partly funded by the EU and is published in the journal Cell. The team's results revealed how yeast cells use a conveyor belt mechanism to offload damaged proteins into the mother cells before dividing into new cells, a process known as mitosis. 'This ensures that the daughter cell is born without age-related damage,' said lead researcher Professor Thomas Nyström from the department of cell and molecular biology at the University of Gothenburg in Sweden. Professor Nyström's research team has published many previous studies of cell ageing, but the new research is the key piece of the jigsaw puzzle. The research was carried out as part of two EU-funded projects, MIMAGE ('Role of mitochondria in conserved mechanisms of ageing') funded with EUR 7.4 million, and PROTEOMAGE ('Functional analysis of evolutionarily conserved mechanisms of ageing on advanced proteome analysis'), funded with EUR 10.7 million under the 'Life sciences, genomics and biotechnology for health' Thematic area of the Sixth Framework Programme (FP6). The research shows how daughter cells use conveyor-belt-like structures to transport the damaged proteins back to the mother cells, thereby allowing the new young cells to be 'born' young and healthy. 'Previously it was believed that these structures allowed only one-way traffic of proteins and organelles from mother cell to daughter cell,' said Professor Nyström. 'We can now show that damaged proteins are transported in the opposite direction. In principle, this means that the daughter cell uses the mother cell as a dustbin for all the rubbish resulting from the ageing process, ensuring that the newly formed cell is born without age-related damage.' The research team discovered that the transportation process is a mechanical one that uses structures called actin cables that operate like conveyor belts. For the actin cables to form properly, a special gene called SIR2 is needed. Previous studies have shown that changing the SIR2 gene can considerably extend the lifespan of an organism. 'Increased SIR2 activity means a longer life, whereas a damaged SIR2 gene accelerates ageing,' said Professor Nyström. 'This has been demonstrated in studies of yeast, worms, flies and fish, and may also be the case in mammals.' Professor Nyström believes that the new findings of how damaged proteins are transported back to mother cells could eventually lead to new treatments for age-related diseases caused by protein toxicity in human beings, although it is currently too early to say how. 'The first step is to study whether this transportation of damaged proteins also occurs in the cells of mammals, including humans, for example in the formation of sex cells and stem cells,' he said. The EU-funded MIMAGE and PROTEOMAGE projects had a total of 31 partners from 12 EU countries plus Canada and China.
Countries
Sweden