Scientists crack rabies virus' armour
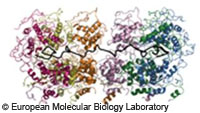
Scientists from the European Molecular Biology Laboratory have cracked the protection shield used by the rabies virus to protect itself from the body's immune system. The discovery represents a new potential avenue of attack for drugs against rabies and similar diseases such as Ebola and measles. Although their symptoms are very different, Ebola, measles and rabies all belong to a class of viruses which carry their genetic information on a single RNA (ribonucleic acid) molecule. In contrast, most other living organisms keep their genetic information on double-stranded DNA (deoxyribonucleic acid). The rabies virus spreads by breaking into human cells, and effectively hijacking the cell's own machinery to produce more copies of the virus, which then go on to infect more of the host's cells. Key to the success of this operation is a viral protein called a nucleoprotein, which wraps itself around the rabies virus' RNA like a protection shield. Without the shield, the virus' RNA would quickly be destroyed by the enzymes of the host's immune system. Researchers from the EMBL and the Institute of Molecular and Structural Virology (IVMS) in Grenoble used high-intensity X-rays to examine crystals of nucleoprotein bound to RNA. From this they were able to produce a striking high resolution image of the protein. The image shows that the nucleoprotein has two domains which clasp around the RNA strand like a clamp. Many nucleoproteins bind side-by-side along the whole length of the RNA molecule, protecting it from attack by the host's immune system. However, whilst enclosed in this armour, the virus is also unable to access the host's machinery, which it needs to replicate itself. The picture of the protein suggests that the part of the protein connecting the two domains could act like a hinge. When the time for replication has come, a certain signal causes the hinge to open up. 'This dynamic mechanism makes nucleoproteins an excellent drug target,' said Rob Ruigrok, Head of the IVMS. 'Small agents that bind to the protein in such a way to block its flexibility and keep it in the closed state, would prevent replication of the virus and would stop it from spreading.' The discovery has implications for the treatment of other viruses which use similar protection systems, including Ebola, measles and Borna virus.