Novel biosensors of antibiotics
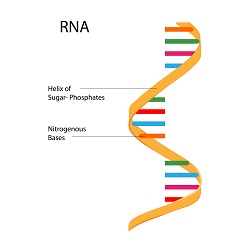
The health and safety risks imposed by the presence of antibiotics in food, drinking water, and environmental waters emphasise the need for continuous monitoring. According to EU regulations, food manufacturers must screen their products for traces of antibiotics. Currently, screening of aquatic pollution by chemicals including antibiotics is performed by liquid chromatographic techniques combined with mass spectrometric analysis. However, antibiotic sensing requires more advanced sensors that would accurately, rapidly and inexpensively report on the presence of antibiotics in various environments. Scientists of the EU-funded DrugSense project proposed to develop innovative sensors that rely on RNA molecules used by bacteria to switch on and off antibiotic resistance genes. RNA aptamers are increasingly being used in biosensor development to bind the analyte of interest with an excellent sensitivity, similar to the affinity exhibited by antibodies. Other approaches for antibacterial detection include the immobilisation of enzymes that break down specific antibiotics such as penicillin, causing a change in the pH of the target sample, as well as functionalised gold nanoparticles. Exploiting anti-bacterial mechanisms “The DrugSense concept relies on the well-established mechanisms evolved in bacteria to overcome the antibiotic imposed pressure,″ explains project coordinator Prof. Rotem Sorek. These mechanisms include enzymatic degradation of antibiotic molecules, efflux from the cells via specific pumps or protection of antibiotic targets via appropriate chemical modifications. However, antibiotic resistance often comes with a cost to bacterial fitness. As a result, bacteria employ regulatory mechanisms by which they sense the presence of antibiotics and selectively activate the expression of the relevant resistance genes only during antibiotic exposure. Growing evidence indicates that for gene regulation bacteria employ in addition to classic transcription-factors, cis-regulatory non-coding RNAs (ncRNAs) as antibiotic sensors. Previously, the DrugSense team through genome-wide studies had identified RNA molecules - known as ribo-regulators - that respond to the presence of antibiotics by stalling ribosomes thus controlling the expression of antibiotic resistance genes. This mechanism seems to be functional in both pathogenic and commensal bacteria. Innovative biosensors based on bacterial RNA molecules “These ribo-regulators can thus function as efficient antibiotics sensors″ outlines Prof. Rotem Sorek and “we will use them to develop a prototype for a highly sensitive bio-sensor, capable of rapid detection of trace levels of multiple antibiotics in food, water and other substances in a cost-effective manner.″ During DrugSense scientists utilised their earlier discoveries to bio-engineer the antibiotics sensor. At the same time, they performed comprehensive market research to identify the needs, map the competition and pinpoint market segments where the biosensor would offer certain advantages over existing products. In view of the future, Prof. Sorek hopes to “achieve an IP protected proof of concept prototype that will attract further external investments, and help bring our technology to the market.″
Keywords
DrugSense, antibiotic, bacteria, resistance, biosensor, RNA