Monitoring drug-levels in transplant patients
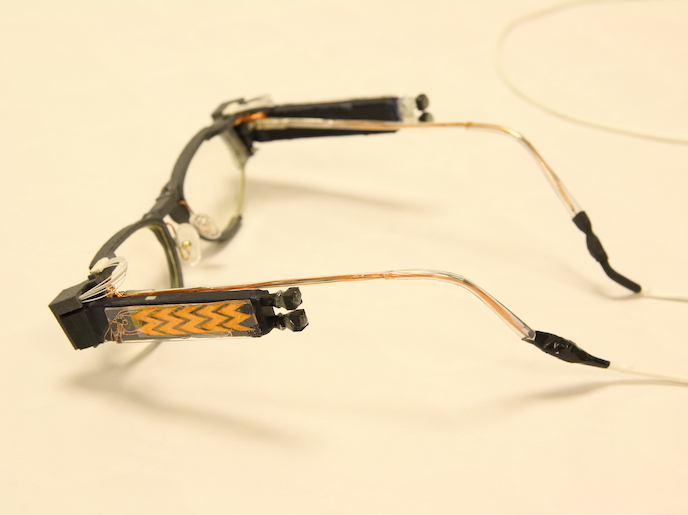
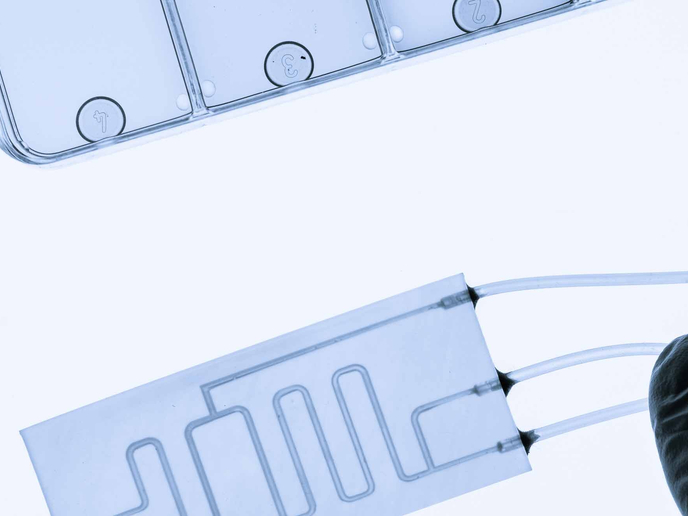
How do we make sure transplant patients are receiving the right medication levels? If levels of immunosuppressive drugs are too low, there is a danger of organ rejection, but too high and the patient may be unable to fight off infections. The NANODEM project has come up with a new device to monitor drug levels in transplant patients. ‘At the heart of the device is a biochip that measures the concentration of immunosuppressants in a patients blood,’ explains project coordinator, Francesco Baldini from the Institute of Applied Physics in Florence, Italy. ‘The miniaturisation of the device and minimally-invasive sampling approach allows therapeutic drugs to be monitored more accurately and at shorter time intervals than existing methods.’ Currently, immunosuppressant drug levels are monitored by standard blood tests, but they don’t provide information quickly enough or give detailed information about the drugs’ activity in the patient, which varies. ‘Our point-of-care testing (POCT) device is a big step forward. It allows frequent monitoring close to the patient, without samples being sent to a central laboratory,’ says Baldini. The NANODEM team is made up of academics and SMEs from 5 EU countries and has used expertise from chemistry, biochemistry, optics and medicine as well as micro and nanotechnologies in their design. Their device is able to measure very low immunosuppressant drug concentrations, in the order of picograms per millilitre and in addition, detects only the free drug concentration - the relatively small fraction (2-8 %) of the drug in the blood stream not bound to proteins, considered more closely related to the drug’s efficacy and also toxicity. Using an intravenous microdialysis catheter, a patient’s sample can be continuously drawn and mixed with the necessary reagents in microchannels, each devoted to the detection of a single immunosuppressant. ‘In this way it is possible, using few microliters, to simultaneously measure multiple substances, which is important from a clinical point of view,’ explains Baldini. The system developed by NANODEM is extremely efficient and can achieve very low detection limits. Drug molecules are first captured on the surfaces of antibody coated polystyrene nanoparticles containing magnetic granules and fluorescent molecules. The antibody molecules are able to capture a specific immunosuppressant drug and on reaching a sensing layer, the fluorescent nanoparticles fluoresce, illuminated by a light beam. Being also magnetic, an applied magnetic field speeds up their arrival at the sensing surface. So far detection limits have been achieved for two important immunosuppressants drugs: cyclosporin A and mycophenolic acid. Another important achievement, adds Baldini, has been making the biochip regenerable - so a patient can use it continuously for multiple readings with the final target of a measurement over a 48 hour period. Tests showed the device could be regenerated for thirty measurement cycles. For the future Baldini says, there is a considerable interest in the technology from the large-scale medical industry. Currently trials are being planned at the Klinikum rechts der Isar hospital of the Technical University of Munich (TUM) to investigate the importance of the free fraction in patients after kidney transplantation. ‘These trials will make use of the body interface module developed within the project and will allow us to compare our new device to standard laboratory methods and we are confident that we can show its enhanced performance addresses a clear medical need,’ concludes Baldini.
Keywords
NANODEM, nanophotonics, therapeutic drug monitoring, immunosuppression, point-of-caretesting, microfluidics, nanoparticles