Re-education for the immune system to fight diabetes
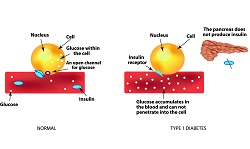
Currently, there are more than half a million type 1 diabetes sufferers in Europe, a disease with no cure or means to prevent its onset. Destruction of insulin-producing beta cells is a gradual process and most patients at diagnosis still have some residual insulin production. Evidence from the Diabetes Control and Complications Trial (DCCT) indicated that individuals with residual insulin had better glycaemic control, reduced rates of hypoglycaemia and lower risk for long term complications. Most treatment approaches are designed to efficiently replace the low or absent insulin levels in patients. However, the low efficiency rate of this strategy suggests that preservation of even small amounts of endogenous insulin production could serve as an alternative to improving glycaemic control. This will not only reduce hypoglycaemia but also long-term complications such as impaired vision, kidney failure and foot ulcers. Scientists of the EU-funded EE-ASI project proposed an enhanced epidermal antigen specific immunotherapy (EE-ASI) approach to re-educate the immune system to stop destroying pancreatic beta cells. This innovative system delivers peptides present only on beta cells in gold nanoparticles. As project co-ordinator Prof. Dayan explains, ‘The nanoparticles are efficiently taken up by dendritic cells leading to presentation of the peptide in a non-inflammatory context, which promotes regulatory T cell generation.’ A second tolerogenic payload such as Interleukin-10, in addition to antigen, is included to promote regulatory T cell production. Nanoparticle distribution Although the EE-ASI approach resembles vaccination, it aims to switch off autoimmune responses by activating regulatory T cells. These are known for recognising self-proteins and acting protectively to suppress any attempts by the body to develop immune responses to self-proteins. In EE-ASI, partners loaded the proinsulin peptide C19-A3 into gold nanoparticles with very high efficiency. ‘Gold is very inert and has anti-inflammatory properties that seem to be beneficial,’ Prof. Dayan continues. When delivered ex vivo on human skin with microneedles, these peptide-loaded nanoparticles diffused into the epidermis and were taken up by Langerhans cells. To do so, their size had to be five nanometres in diameter as bigger particles do not follow this diffusion pattern. Pre-clinical experiments in mice demonstrated diffusion to distant lymph nodes more rapidly than peptide alone. Toxicology studies validated the safety of the approach and led to a patent application and a phase I clinical study in under four years since the start of the project. Clinical efficacy The EE-ASI clinical study represents the first in-man study on the effects of delivering a proinsulin peptide conjugated to nanoparticles. Results revealed that when given intradermally, the gold stays in the skin for more than six months along with a local lymphocytic infiltrate that warrants further investigation. Additional studies will also determine if the peptide remains associated with the gold. Furthermore, the consortium is ‘exploring other tolerogenic molecules as well as incorporation of DNA molecules to express self-peptides in the skin,’ Prof. Dayan points out. Although efficacy studies are pending, encouraging the body to recognise insulin and stop its immune destruction seems like a valid approach for treating type 1 diabetes. Given the ease of application, the EE-ASI approach promises better patient compliance and fewer complications than hormone replacement therapy. In turn, this will lead to a better clinical outcome and an improved quality of life for diabetes patients and their families.
Keywords
Immune system, immunotherapy, type 1 diabetes, insulin, pancreatic beta cells, EE-ASI, nanoparticle, regulatory T cells, Interleukin 10, proinsulin