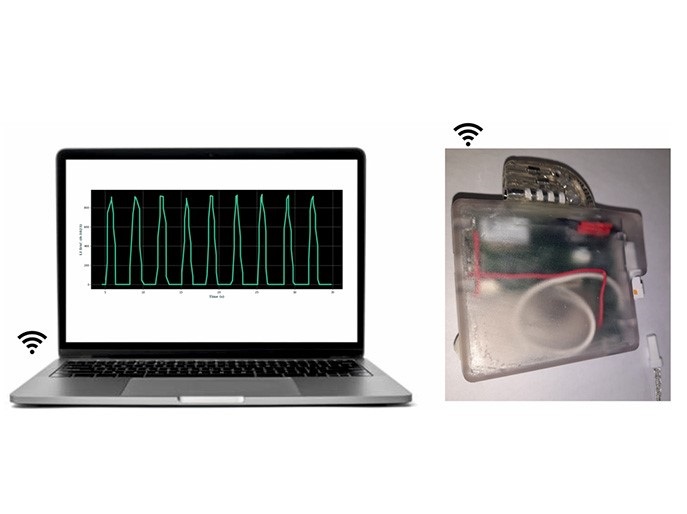
Neuronal-based heart pacemaker
In healthy humans, heart rate varies within the respiratory cycle causing the heart to beat a little faster during breathing than during the exhalation phase. This modulation of the heartbeat frequency by breathing is termed respiratory sinus arrhythmia, and its decline is an early prognosis of chronic heart disease, such as chronic arrhythmias or heart failure. Restoring beat-to-beat cardiac variability has been hypothesised to provide an important therapy for these diseases, which are not adequately addressed by existing therapies. Therefore, a device that responds to physiological feedback in a similar way to respiratory neurons could potentially provide a valuable treatment option for chronic heart disease.
A pacemaker that replicates natural heartbeat
The EU-funded CResPace project developed a novel pacemaker that addresses this paradigm by resynchronising heart chambers while restoring the adaptation of heart rate and heart chamber delays to physiological feedback on a beat-to-beat basis. The function of the CResPace pacemaker differs from a conventional heart pacemaker that works by providing steady pace electrical stimulation to the heart muscle to maintain a regular heartbeat. The technology replicates the function of neural circuits located at the base of the brain known as central pattern generators, which control respiration and heart rate. “Our pacemaker resynchronises the contraction of heart chambers in response to changes in breathing patterns, arterial oxygen concentration, arterial carbon dioxide and blood pressure to revert chronic cardiac disease,” explains project coordinator Alain Nogaret. To achieve this, the team designed arterial gas sensors and blood pressure sensors that feed this information into the pacemaker in real time. These were miniaturised and are expected to find applications in a wider range of bioelectronic devices for monitoring and adapting to physiological feedback.
Computational tools for taming neuronal dynamics
A major challenge and bottleneck to implementing neuronal electronics in bioelectronic devices was to condition artificial neuronal circuits to provide the correct adaptation to the physiological inputs. For this, researchers developed sophisticated computational techniques that obtain the optimal circuit parameters to replicate the adaptation of the healthy heart. Besides providing the correct adaptation, neuronal circuits have the unique ability to resynchronise biological rhythms thanks to the nonlinear response of neurons. This saves the diseased heart energy, and in combination with adaptation, can produce major improvement in cardiac function in models of disease. “The prospect of stopping the progression of these fatal cardiac diseases and curing them is a major achievement of the project,” emphasises Nogaret.
Pacemaker validation
The heart pacemaker has been validated in its operational environment by restoring cardiac function and placing it under respiratory modulation in models of disease. The next stage of development is to make the technology ready for human use and compliant with CE marking. The basis of the technology can extend to other diseases for restoring normal function of affected organs. Moreover, the CResPace approach provides the groundwork for neuromorphic engineering as it faithfully recapitulates the native neuronal dynamics onto a chip.
Keywords
CResPace, heart pacemaker, neuronal circuits, respiratory sinus arrhythmia, central pattern generators