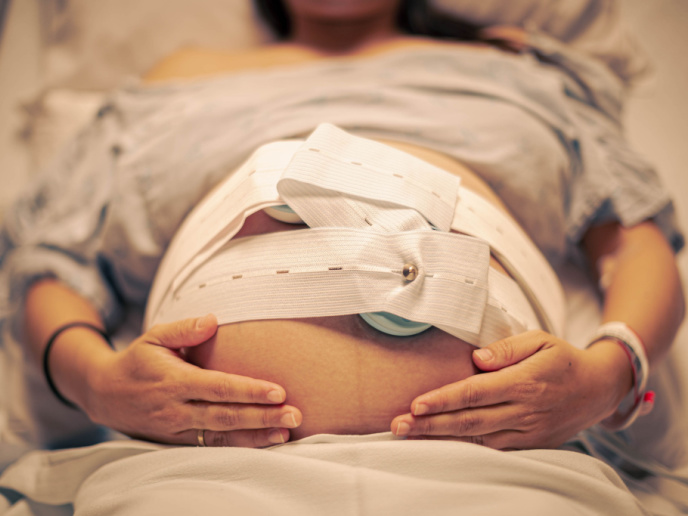
New-generation cardiotocographs: A self-monitoring belt for pregnant women
Traditionally, foetal monitoring takes place with cardiotocographs (CTGs). However, CTGs haven’t shown any major innovation since the 1960s and CTG-related issues are observed in 25 % of foetal deaths. With an increase of aggravating factors in at-risk pregnancies, there is an urgent medical need to improve foetal monitoring while addressing healthcare costs.
A mobile device for monitoring FHR
To address this issue, the EU-funded ARFM project developed a mobile FHR automatic recording (RCF) solution. “Our goal is to facilitate patient self-monitoring at home thanks to an automated detection of FHR through a mobile device,″ explains project coordinator and NATEO Healthcare CEO Philippe Constant. Conventional CTGs are difficult to place for some patients, not always reliable and require medical staff nearby for data acquisition. The ARFM device comes as a multi-sensor belt that is placed on the maternal abdomen to cover the area of foetal cardiac projection. The belt is comfortable to use and can be adapted to a wide variety of patient morphologies. Using AI algorithms, the device searches and tracks FHR automatically, avoiding breaks in RCF tracing. This means that the baby is constantly monitored even when it changes position. It monitors uterine activity and maternal heart rate. Importantly, it can discriminate the foetal from the maternal heart rhythm, guaranteeing reliability of the RCF tracing for the medical team. The device comes in two forms: a hospital continuous monitoring solution that can also be used during childbirth, and a homecare device for remote monitoring. The homecare ARFM CTG version can be used from the 24th week of pregnancy and frequency of measurement readings can be adjusted. It is connected to an obstetric remote monitoring platform and can also be linked to other devices such as those measuring weight, glucose levels and blood pressure. Furthermore, this telemedicine approach offers high-risk pregnancies an alternative to conventional hospitalisation and frees up medical time for midwives.
Device optimisation and future prospects
ARFM partners have performed studies to refine regulatory, clinical and market access strategy for the mobile FHR monitoring device. After taking into consideration the new EMA regulations for medical devices, they will proceed with a new clinical study in order to obtain CE marking. A previous clinical study conducted on 769 patients helped towards device optimisation to cover 95 % of the cardiac projection area. For market access, a study has been conducted and, as a result, partners are being identified both in digital telemedicine technology and current foetal monitoring systems in different European countries. Preliminary market analysis has demonstrated a favourable market for a mobile FHR monitoring device, as well as, further analysis of barriers to entry and market opportunities. “The funding of the ARFM project was paramount for our organisation at this stage of development,″ emphasises Constant. Overall, the ARFM disruptive CTG technology is expected to improve the monitoring of foetal well-being and enhance neonatal outcomes. It also has the potential to decrease healthcare costs related to at-risk pregnancies by 50 %.
Keywords
ARFM, FHR, CTG, mobile device, foetal monitoring, cardiotocograph, pregnancy, neonatal, telemedicine, foetal heart rate