Diagnostic kit for inflammatory bowel disease diagnosis
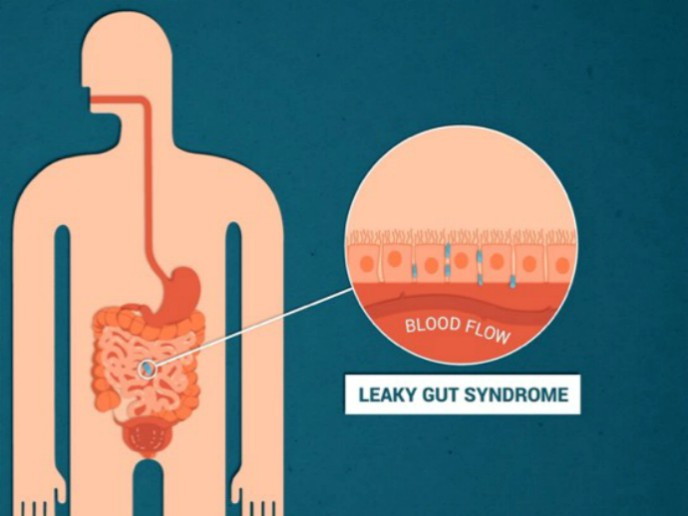
Presently, diagnosis of IBD relies on the assessment of indirect parameters like general inflammation markers in plasma and stool. Patient evaluation is restricted to very invasive methods like biopsy or qualitative means like MRI and microcapsule colonoscopy. Being able to assess lesions in the intestinal wall, characteristic of a pre-IBD condition known as leaky gut syndrome (LGS), would facilitate early treatment and improve patient outcome. Non-invasively assessing intestinal lesions Under physiological conditions, intestinal cells form a physical barrier helping the gut to tightly control every absorption process. In patients with LGS and IBD, these cell connections are lost, compromising digestion products exchange control, enabling bacteria and toxic products to leak into the blood stream. To help clinical decision making, the EU-funded PermeAbility project developed a new proprietary test to measure intestinal permeability, a key parameter for determining intestinal integrity and proper function. “The idea was to develop a non-invasive test that could help identify patients at high risk of IBD,″ explains project coordinator João Silva. The PermeAbility test uses a molecule that is totally safe and does not biologically interact with any part of the gastrointestinal tract when ingested. If there are intestinal wall lesions, the molecule will reach the bloodstream and get cleared in urine within six hours. Urine testing for the presence of the ingested compound provides a very sensitive measure to accurately assess the extent of intestinal permeability. This value is proportional to the existence of intestinal lesions. Although clinical validation is pending, preclinical models provide promising results on test efficacy. Using animals with different levels of intestinal permeability, the team could detect intestinal lesions while demonstrating the safety of the product. The future of the PermeAbility test According to Silva, the most significant achievement was the strategic plan settlement towards certification and market approval, given the borderline features between a drug and a device for the PermeAbility test product. After overcoming regulatory challenges in product classification, partners have devised a business strategy and are currently seeking investment funding. The high sensitivity and non-invasive nature of the test, as well as its easy and user-friendly implementation, highlight the advantages of the test compared to existing methodology. Importantly, its low cost represents an attractive feature that will contribute to it becoming part of the battery of routine tests in national health programmes. There are over 1.9 billion people worldwide at risk of early lesions and 4 million patients with IBD that remain undiagnosed. At the same time, 35 % of intestinal surgeries are unnecessary incurring a high healthcare burden. “The test is an excellent asset to screen, diagnose and monitor disease onset and progression,″ continues Silva. As it can detect micro lesions, it is possible to diagnose intestinal malfunction at a very early stage before IBD development. The PermeAbility test is a novel solution for directly measuring the characteristic lesions of LGS and IBD to minimise unnecessary surgery costs. Importantly, detection of early stage disease will contribute to prompt preventive or therapeutic treatment, reducing evolution to chronic disease, which constitutes one of the problems of westernised countries.
Keywords
PermeAbility, test, inflammatory bowel disease (IBD), leaky gut syndrome (LGS), intestinal permeability, urine