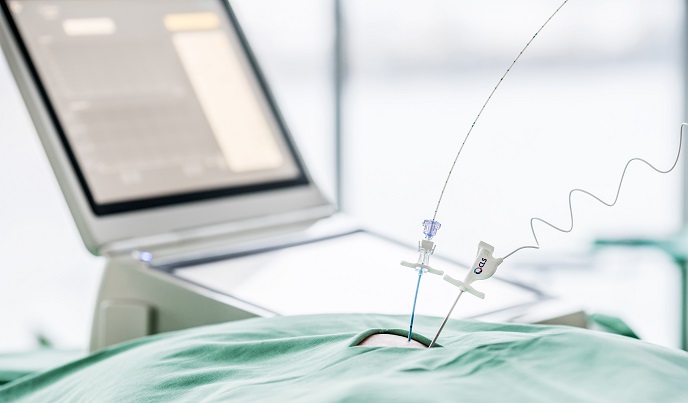
Exploring a unique laser immunotherapy against cancerous tumours
Our immune systems protect us by producing antibodies to fight infections. Drug-based immunotherapies boost these natural defences against illness such as cancer. But with many patients not responding to these immunotherapies, new approaches are needed. The EU-supported INTHER project developed an innovative device to deliver minimally invasive immunostimulating Interstitial Laser Thermotherapy (imILT). The therapy works by attacking the tumour directly and stimulating the patient’s own immune system to attack other (same-type cancer) tumours. The effectiveness of the method was tested against solid pancreatic and breast cancer tumours confirming the therapeutic promise of the new method. The clinical uptake of imILT will reduce the treatment costs of treating solid tumours, such as incurred by surgery. Testing for safety and equipment usability The imILT laser light heats the cancerous tumour in a gentle and controlled way, killing it within a few days and causing it to leak antigens. These antigens activate the body's immune system, which also targets other tumours and metastases in the body. In the case of pancreatic cancer, five patients with stage III pancreatic cancer were treated in France using imILT during open surgery. The patients tolerated the treatment well and the equipment was found to be highly usable and safe. In Portugal, four patients with locally advanced stage III and stage IV pancreatic cancer were also treated using open surgery. The therapy was well tolerated by the patients and no serious side effects resulted from the therapy. As of November 2018, five of the patients who received imILT had a median survival time of 17 months, compared with the published results for locally advanced, inoperable pancreatic cancer, with a median survival of under a year. As for breast cancer, two patients in the UK were treated with imILT. The treatment was well tolerated without serious side effects, and both patients were still alive when followed-up, two and 10 months (respectively), after the therapy. “These tests demonstrate that imILT treatment is safe, non-invasive with few side effects, and at a lower cost per patient compared to surgery or chemical treatment,” says Mr Lars-Erik Eriksson, CEO at CLS. As the treatment happens at a low temperature (about 46 degrees for 30 minutes), the tumour's antigenic proteins are preserved, further stimulating an immunological defence and potentially offering long-term protection against tumours of the same kind of cancer: a possibility still to be properly investigated. Towards clinical practice Currently, CLS and the two hospitals that conducted the pancreatic cancer study are collaborating further on using imILT. Results are to be jointly presented at the ECIO conference in April 2019. Additionally, the Institute of Oncology in Portugal will continue to offer imILT to patients with pancreatic cancer making it the first European hospital to routinely offer it. The TRANBERG delivery system (the laser machine and instruments used to treat the patient, measuring and controlling laser temperature) is CE-marked and market ready, while the imILT method (the treatment protocol) needs to be further verified with more patients. The prevalence of pancreatic cancer is increasing significantly and lacks effective treatments. Additionally, most patients are diagnosed quite late, due to somewhat indeterminate symptoms. “If CLS can offer an alternative for these patients, improving overall survival rates, this will be a milestone. imILT can also be combined with other treatments – an area we will further explore”, says Mr Eriksson.
Keywords
INTHER, immune system, laser, breast cancer, pancreatic cancer, immunotherapy, treatment, antigen, tumour, oncology, metastases