Therapeutic device for obstructive sleep apnoea
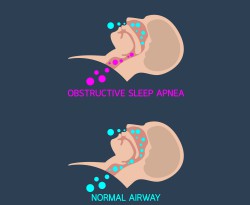
In obstructive sleep apnoea, the respiratory path becomes blocked during sleep when throat muscles intermittently relax. This stops the patient breathing and this condition is accompanied by severe co-morbidity issues. The first line of treatment to keep the airways open during sleep is the use of continuous positive airway pressure (CPAP). However, major discomfort results in poor patient compliance leading to only 50 % treatment success. The EU-funded Therapnea project developed an innovative device to address the unmet needs of patients who cannot comply with current therapies. The main aim was to provide effective therapy even to sleep apnoea patients who can’t be helped with surgery. Initially, the long-term plan was for the Therapnea device to become the first line of treatment for sleep apnoea. Novel device design The Therapnea device operates by providing mechanical support to the walls of the pharynx and the back of the tongue, eliminating partial or complete airway obstruction during sleep. “Our device employs support structures in the upper airways to prevent their collapse during sleep,” explains project coordinator Mr Avi Lior. Researchers had to address various challenges in the design of the device regarding the shape, size and deployment mechanism alongside parameters of the support element in the users’ airways. Following extensive research and development as well as experimentation with various designs, a very thin, bio-compatible, low friction, highly elastic yet durable coating was applied on the support element. “Unlike any other, the Therapnea device has fewer side effects,″ Lior points out. The Therapnea device does not use compressed air, so it is much less annoying, has fewer side effects, does not require a mask and hose connected to a bed-side unit and is quiet. Instead it uses a dental plate carrying a collapsible airways support, a chest-worn driver and a separate bedside unit for device cleaning and setting up during the day. On top of providing therapy, the device also collects large amounts of data about the patients’ respiration and sleep and uploads them to the cloud for remote supervision and analysis. Clinical effect The device was tested in a proof of concept clinical study in India involving 10 patients. Results clearly demonstrated the resolution of gag reflex, the main problem that prohibits the use of support elements in the upper airways. Importantly, a dose-dependent reduction in the severity of apnoea was seen, with one patient demonstrating complete recovery. Prior to device market release expected to take place in 2020, Therapnea researchers plan to run follow-up studies with more patients and for longer periods to finalise the design of the device. “We don’t expect to replace CPAP, as it is the industry standard and treatment of choice for many years,″ emphasises Lior. “We do, however, see a huge need with patients who can’t or won’t tolerate CPAP, suffering severe health consequences from their condition.″ Considering that the sleep apnoea devices market is estimated to be 3 billion dollars, there is definitely a niche for the Therapnea device. So far, the clinical results support its therapeutic efficacy, rendering it a potential game changer in the field.
Keywords
Therapnea, device, airway, obstructive sleep apnoea, continuous positive airway pressure (CPAP)