The IMI EHDEN Consortium Launches Its First Open Data Partner Call
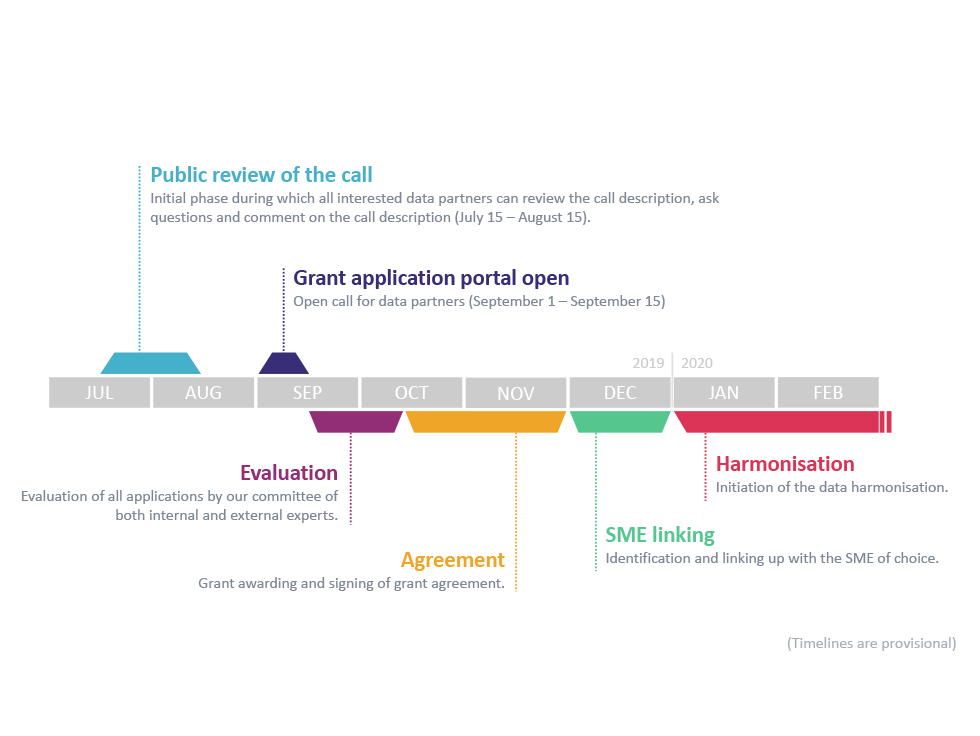
EHDEN (https://www.ehden.eu/) is a pan-European initiative funded by the IMI2 (Innovative Medicines Initiative) (https://www.imi.europa.eu/). Its main objective is to address the current challenges in generating insights and evidence from real-world clinical data at scale, to support patients, clinicians, payers, regulators, governments, and the pharmaceutical industry in better understanding well-being, disease, treatments, outcomes and new therapeutics and devices through a federated network of many million patient data records. The ambitions of the EHDEN project are high. We aim to standardise more than 100 million patient records across Europe from different geographic areas and different data sources over the coming five years. Mapping of healthcare data to the OMOP-CDM will facilitate the re-use for a variety of purposes, enhancing and accelerating research and healthcare decision-making for global benefit. The EHDEN Consortium would like to invite all Data Custodians to participate in this endeavour by becoming a Data Partner in the EHDEN community. Data Custodians can benefit from seed funding that will be made available via a €17 million “Harmonisation Fund” that subsidises conversions to the OMOP-CDM performed by EHDEN-certified SMEs. To this end, EHDEN is organising its first open Data Partner Call from 15 July - 15 September, 2019. • From 15 July - 15 August, the call description is available for public review. This enables the EHDEN project to optimise the procedures and to clarify the pilot call text if deemed necessary. • From 1 - 15 September, the Grant application portal will open and we will accept Data Partner applications. By becoming a Data Partner in the EHDEN community you will: 1. Facilitate scientific collaboration, and the connection with peers and their learnings by becoming part of a thriving academic/medical network and community. 2. Boost opportunities to participate in international studies for larger academic impact. 3. Improve interoperability; expand the value of your data by enabling its re-use across a wide range of analytic use cases. 4. Receive financial support for uptake, extension and validation of mappings (EHDEN grants). 5. Increase visibility for your data, expanding opportunities to be approached for sponsored studies helping long-term sustainability of the data source with stable revenue streams. 6. Increase capability for analysis thanks to a host of open source tools. Quickly and consistently design and perform studies (cohort definition, etc.). 7. Increase transparency of analyses and reproducibility, enabling studies to be easily ‘transposed’ to other data sources for multiplied impact (e.g. in publications). 8. Perform studies faster. If this exciting prospect interests you, please contact us via applicants@ehden.eu or visit www.ehden.eu for more details. The EHDEN Consortium is looking forward to your potential application and to collaborate with you.
Keywords
Healthcare, data, OMOP, Common data model, harmonisation, community, outcomes, patients