A multimodal novel lensless microscopy technology for medical applications
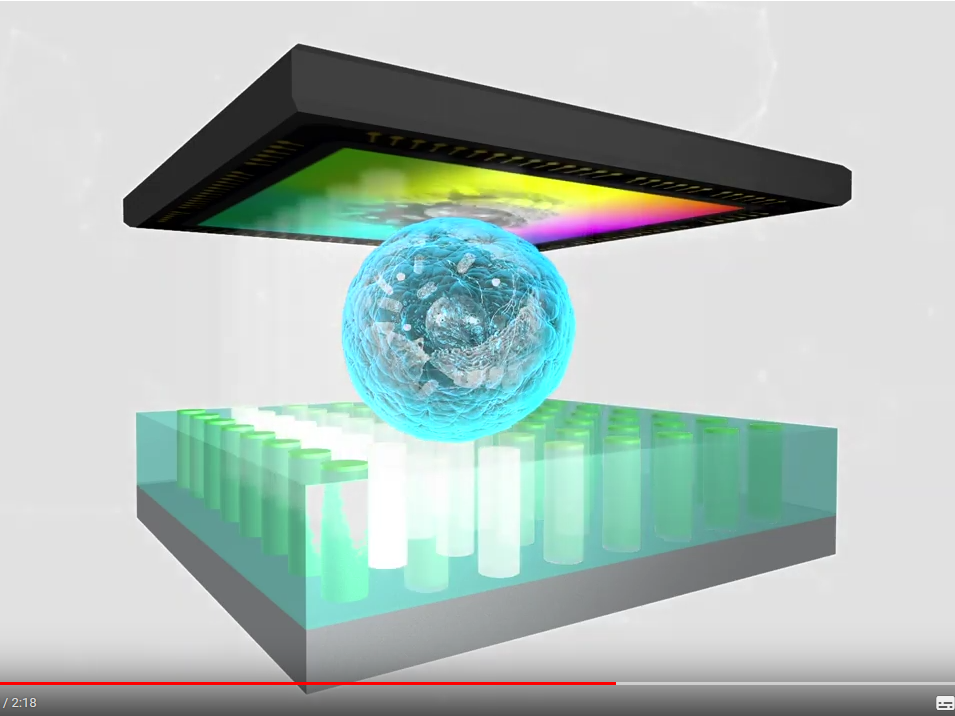
Today's state-of-the-art analysis of biological samples by light microscopy includes a vast variety of techniques ranging from conventional bright field microscopy and phase contrast microscopy to high resolution confocal laser scanning microscopy and to recently developed super resolution microscopy techniques like stimulated emission depletion (STED) or stochastic optical reconstruction microscopy (STORM) which abnegate Abbe's limit of diffraction. Despite the availability of these sophisticated, super resolution techniques, reproducible visualization of cells and identification of subcellular structures in biological samples still requires staining with dyes or immunolabeling by antibodies to specific cellular antigens. Generally, in-vitro observation of living cells can provide valuable insights into their structure and dynamics including organization of organelles and transduction of chemical signals involved in cell–cell and cell–matrix interactions. Unfortunately, there is a limited use for long term in-vitro imaging as most high-resolution microscopy technologies require processed/fixed tissues or cells. As both high resolution optical microscopy and fluorescence imaging usually require highly skilled users, expensive equipment and maintenance, the presented novel digital in-line holographic microscopy (DIHM) in-vitro imaging technology opens a vast field of applications for standard users. This analytical optical system offers quick and reproducible results at low costs. Moreover, it voids the necessity of referral to specialized labs and is easily implemented as a diagnostic tool for doctors (general practitioners and specialists). DIHM is based on the numerical reconstruction of a digitally recorded hologram. It allows for the acquisition of both, the amplitude and phase information of a wave front shaped by the microscopic sample. The advantage of the DIHM lies in the simplicity of its setup: the microscope consists of a light-emitting diode (LED) as an illumination source, appropriate filtering for coherence enhancement and an image sensor. The comprehensive data processing algorithm transforms the recorded holograms into a microscope image by angular spectrum approach and digital filtering [1]. In general, the resolution of such a microscope is strongly influenced by the spatial coherence length of the illumination, which can be enhanced via reducing the emitting area, either by cutting a part of the wave front with the pinhole or by use of a point-like nanoLED. The nanoLED arrays developed within the EU Horizon 2020 program ChipScope project will allow enhancement of the imaging resolution compatible to the conventional optical microscopy. Lensless DIHM microscope This fact makes lensless microscopy an ideal tool for medical diagnosis in remote areas since there is no need for the medical doctor to bring and maintain large, heavy and sensitive analysis devices. A simple laptop and a suitcase sized lensless microscope assembly is enough to – for example – make a parasite diagnosis from body fluid samples (e.g. Malaria, Amoeba etc.). The robust construction enables a fast, reliable and automated analysis of the specimen by combining not only high-resolution light microscopy but also implementing modern analysis techniques based on the detection of changes in human DNA, identifying viral genomes and immunological characterization in one device. To provide the highest light sensitivity and optical resolution, the system is equipped with a normal grayscale camera to work in a multi-cell imaging bright field mode. This novel lensless microscope is equipped with a microfluidic flow channel system for handling living cells and imaging. [1] Scholz G, Mariana S, Dharmawan A, Syamsu I, Hörmann P, Reuse C, et al. Continuous Live-Cell Culture Imaging and Single-Cell Tracking by Computational Lensfree LED Microscopy. Sensors. 2019 Mar 11; 19(5): 1234. http://dx.doi.org/10.3390/s19051234