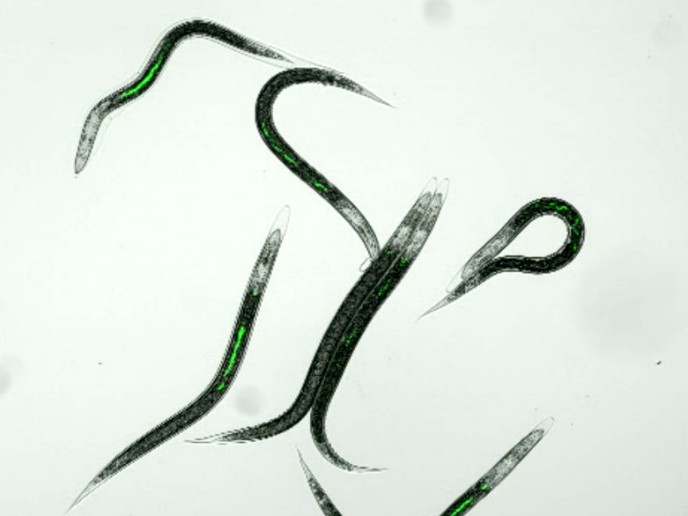
A peek inside nature’s pesticide factory
According to the Food and Agriculture Organization of the United Nations (FAO), approximately one third of the world’s population makes a living from agriculture. This task is complicated by insect pests, which destroy billions of euros worth of crops every year. Chemical pesticides are the primary weapon against these invaders, but they are not environmentally sustainable. Fortunately, nature has provided an eco-friendly solution in the form of roundworms that kill insects. The entomopathogenic nematode (EPN) called Heterorhabditis is commercially available and widely used to control insect pests. However, it may lose its efficacy during storage prior to use. With the support of the Marie Skłodowska-Curie programme, researchers working on the STEPN-UP project have uncovered a signalling pathway critical to the maintenance of entomopathogenic properties that could help preserve or extend shelf-life. Lying in wait together Heterorhabditis is a holobiont, an organism that forms a small ecosystem by hosting various symbionts. In this case, the ‘hostee’ is a bacterial partner, Photorhabdus. As project coordinator David Clarke explains, “Photorhabdus colonise the gut of a special developmental stage of the nematode called the infective juvenile (IJ) and the colonised IJ is in fact the commercially available EPN product.” The IJ invades the larvae of insects and regurgitates the bacteria, killing the insects. The nematode feeds on their decomposing bodies. IJs can remain viable for several months without feeding and it is critical that the gut bacteria remain viable as well. This is essentially the ‘shelf-life’ of the EPN product. However, very little is known about how the bacterial symbionts stay alive and well. Keeping deadly bacteria alive On the hypothesis that the EPN feeds the bacteria, researchers started looking for RNA evidence suggesting active nutritional exchange between the two organisms. They studied transcriptional profiles of IJs colonised with bacteria and compared them to those without. Surprisingly, there was no evidence of exchange. Clarke’s team, including Marie Skłodowska-Curie scholar Dr Dana Blackburn, then turned to the bacteria. According to Clarke, “Using a targeted genetic approach, we identified a signalling pathway in Photorhabdus that is required for the persistence of the bacteria in the nematode.” It turns out the so-called Cpx pathway, well-described in another gut-dwelling bacteria, Escherichia coli or E. coli, must be active for Photorhabdus to remain viable in its host. The STEPN-UP discovery has important implications for increasing the shelf-life of nature’s pesticide Heterorhabditis. Future research could also lead to enhanced food security to feed a growing population. Finally, given that human gut microbiota has been shown to produce a range of small molecules that influences human immune development, results could impact our understanding of human health and disease.
Keywords
STEPN-UP, bacteria, insect, gut, infective juvenile (IJ), pesticide, agriculture, EPN, nematode, Photorhabdus, shelf-life, Heterorhabditis, signalling pathway, pests, entomopathogenic, symbionts, roundworms