Less precious, more efficient hydrogen fuel cells
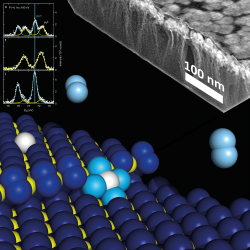
PEMFCs use a water-based, acidic polymer membrane as their electrolyte together with platinum (Pt)-based electrodes. Hydrogen fuel is processed on the surface of a Pt-based catalyst at the anode where electrons are separated from protons. The protons pass through the membrane before reaching the cathode side of the cell. On the cathode side, the precious metal electrode combines protons and electrons with oxygen, and water is produced, which is expelled as the only waste product. Specifically, oxygen can be produced in a purified form or extracted at the electrode directly from the air. The EU-funded project CHIPCAT (Design of thin-film nanocatalysts for on-chip fuel cell technology) was launched to search for a viable alternative to the use of Pt nanoparticles. As a precious metal, Pt increases the price of hydrogen fuel cells, comprising up to 50 % of the manufacturing cost. During the four-year project, researchers explored different aspects of physical deposition technology. Typical industrial electrode-fabrication processes involve wet steps, which are incompatible with the technology of silicon-based electronic devices. 'Platinum doped cerium oxides (Pt-CeOx) thin film catalysts, for which patent applications had been filed before CHIPCAT, were proposed to maximise the efficiency of platinum used in fuel cells', explains Dr Daniel Mazur from the Charles University in Prague, the Czech Republic, a researcher and management secretary of the project. The new thin-film nanocatalysts consist of the Pt atoms, predominantly in ionic state, dispersed within a matrix of a reducible oxide – ceria. The oxide crystallites have diameters of a few nanometres to ensure that the majority of Pt atoms end up on the surfaces of the crystallites and are catalytically active. ‘We had to convince ourselves and others that this compound works as proposed. After that we had to discover the key mechanisms that make these nanocatalysts work, so that we could refine them and find additives to make the best out of it,’ Dr. Mazur recalls. He stresses that ‘a host of experiments on simplified materials and of sophisticated model calculations had to be done to enable reaching the targets of the project.’ Dr Mazur also noted that 'eventually, our published results indicated that the new catalysts can outlast conventional ones, because dispersed platinum atoms hardly coalesce at all and, therefore, keep the original performance so long as the scaffolding (carbonaceous material) holds'. He also added: 'With the use of high-resolution transmission electron microscopy, we were eventually able to visualise the individual Pt atoms embedded in the ceria crystallites. This evidence has long been called for by our scientific peers, who were not convinced by our claim of the "atomically dispersed" nature of the catalyst.' Significant efforts were also devoted to the construction of a miniature fuel cell so that it could be incorporated in an integrated circuit device. Researchers designed a system on a chip, in which hydrogen flows through microfluidic channels etched on a silicon wafer chip. However, 'the different geometry as compared to the sandwich structure of conventional PEMFCs meant that only processes at the channel edges contribute to the performance of the micro-fuel cell. This limits its performance and the amount of power that can be harvested', said Dr Mazur. There are several strategies proposed to overcome this serious barrier, including the catalyst being deposited onto the membrane rather than on the trench walls. CHIPCAT findings are described in more than 60 scientific papers published in high-impact peer-reviewed journals, including Nature Communications and Nature Materials. These publications document the deep understanding obtained of processes taking place in the studied materials and was key to reach the final outcomes. The first steps have also been made towards commercialisation of the new thin-film catalyst technology. 'The Charles University in Prague, the project coordinator in partnership with the Jablotron Group have created a spin-out venture under the leadership of Prof. Vladimír Matolín (holder of the patents mentioned earlier)', said Dr Mazur. The company LEANCAT s.r.o. is already offering the atomic nanocatalysts as a product. At the same time, they are manufacturing and selling fuel cell test stations that were developed at Charles University as part of the CHIPCAT project.
Keywords
Hydrogen, proton exchange membrane fuel cells, single-atom catalysis, platinum, CHIPCAT